Non-HIV Vaccine-Induced Immune Responses as Potential Baseline Immunogenicity Predictors of ALVAC-HIV and AIDSVAX B/E-Induced Immune Responses
- PMID: 39339842
- PMCID: PMC11437453
- DOI: 10.3390/v16091365
Non-HIV Vaccine-Induced Immune Responses as Potential Baseline Immunogenicity Predictors of ALVAC-HIV and AIDSVAX B/E-Induced Immune Responses
Abstract
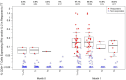
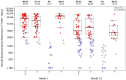
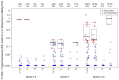
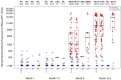
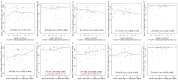
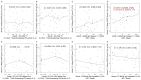
Identifying correlations between immune responses elicited via HIV and non-HIV vaccines could aid the search for correlates of HIV protection and increase statistical power in HIV vaccine-efficacy trial designs. An exploratory objective of the HVTN 097 phase 1b trial was to assess whether immune responses [focusing on those supported as correlates of risk (CoR) of HIV acquisition] induced via the RV144 pox-prime HIV vaccine regimen correlated with those induced via tetanus toxoid (TT) and/or hepatitis B virus (HBV) vaccines. We measured TT-specific and HBV-specific IgG-binding antibody responses and TT-specific and HBV-specific CD4+ T-cell responses at multiple time points in HVTN 097 participants, and we assessed their correlations at peak time points with HIV vaccine (ALVAC-HIV and AIDSVAX B/E)-induced responses. Four correlations were significant [false discovery rate-adjusted p-value (FDR) ≤ 0.2]. Three of these four were with IgG-binding antibody responses to TT measured one month after TT receipt, with the strongest and most significant correlation [rho = 0.368 (95% CI: 0.096, 0.588; p = 0.008; FDR = 0.137)] being with IgG-binding antibody responses to MN gp120 gDneg (B protein boost) measured two weeks after the second ALVAC-HIV and AIDSVAX B/E boost. The fourth significant correlation [(rho = 0.361; 95% CI: 0.049, 0.609; p = 0.021; FDR = 0.137)] was between CD4+ T-cell responses to a hepatitis B surface antigen peptide pool, measured 2 weeks after the third HBV vaccination, and IgG-binding antibody responses to gp70BCaseAV1V2 (B V1V2 immune correlate), measured two weeks after the second ALVAC-HIV and AIDSVAX B/E boost. These moderate correlations imply that either vaccine, TT or HBV, could potentially provide a moderately useful immunogenicity predictor for the ALVAC-HIV and AIDSVAX B/E HIV vaccine regimen.
Keywords: HIV vaccine; T-cell responses; binding antibodies; biomarkers; immune correlates.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Plotkin S.A., Gilbert P.B. Correlates of Protection. In: Orenstein W., Offit P., Edwards K., Plotkin S., editors. Vaccines. 8th ed. Elsevier Inc.; New York, NY, USA: 2022. pp. 35–40.
-
- King D.F., Groves H., Weller C., Jones I., Cramer J.P., Gilbert P.B., Goldblatt D., Gruber M.F., Kampmann B., Maïga D., et al. Realising the potential of correlates of protection for vaccine development, licensure and use: Short summary. npj Vaccines. 2024;9:82. doi: 10.1038/s41541-024-00872-6. - DOI - PMC - PubMed
-
- Cavaleri M. How Can Correlates of Protection Help the Regulatory Process for Licensure of New Vaccines. Presented for the Product Development for Vaccines Advisory Committee (PDVAC) of the European Medicines Agency, 12 December 2023. [(accessed on 17 July 2024)]. Available online: https://cdn.who.int/media/docs/default-source/immunization/pdvac/pdvac-2....
-
- Gilbert P.B., Fong Y., Hejazi N.S., Kenny A., Huang Y., Carone M., Benkeser D., Follmann D. Four statistical frameworks for assessing an immune correlate of protection (surrogate endpoint) from a randomized, controlled, vaccine efficacy trial. Vaccine. 2024;42:2181–2190. doi: 10.1016/j.vaccine.2024.02.071. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA277133/CA/NCI NIH HHS/United States
- R37AI054165/National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH)
- R37 AI054165/AI/NIAID NIH HHS/United States
- UM1 AI068635/AI/NIAID NIH HHS/United States
- UM1AI068635/National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH)
LinkOut - more resources
Full Text Sources
Medical
Research Materials

