Serologic Cross-Reactivity between the Mumps Virus Vaccine Genotype A Strain and the Circulating Genotype G Strain
- PMID: 39339910
- PMCID: PMC11437446
- DOI: 10.3390/v16091434
Serologic Cross-Reactivity between the Mumps Virus Vaccine Genotype A Strain and the Circulating Genotype G Strain
Abstract
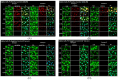
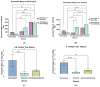
Recent mumps outbreaks have been observed in vaccinated young adults due to the mumps virus (MuV) of genotype G, whereas the current vaccine is a mixture of two genotype A strains. These outbreaks could be attributed to waning vaccine immunity or the antigenic differences between the HN and F glycoproteins in the vaccine and circulating MuV. These glycoproteins are essential targets for the immune system, and antigenic variations may reduce the recognition of mumps antibodies, rendering the population susceptible to the MuV. We established stable cell lines expressing the MuV glycoproteins to study cross-reactivity between genotype A and genotype G. Cross-reactivity between the genotypes was evaluated via immunofluorescence using patient sera from vaccinated individuals, infected individuals, and vaccinated individuals infected with genotype G. Titer ratios showed that the vaccinated individuals exhibited a titer 3.68 times higher for the HN protein and 2.3 times higher for the F protein when comparing genotype A with genotype G. In contrast, the infected individuals showed a lower titer for genotype A compared with genotype G, at 0.43 and 0.33 for the HN and F proteins, respectively. No difference in titer ratio was observed for individuals vaccinated and subsequently infected with mumps. These findings suggest that antigenic variations between the two genotypes may potentially result in immune escape of the circulating strain, resulting in individuals susceptible to the MuV.
Keywords: antigenic variation; cross-reactivity; genotype A; genotype G; immunofluorescence; mumps virus; serology.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

