Coxsackievirus B3 Activates Macrophages Independently of CAR-Mediated Viral Entry
- PMID: 39339932
- PMCID: PMC11437450
- DOI: 10.3390/v16091456
Coxsackievirus B3 Activates Macrophages Independently of CAR-Mediated Viral Entry
Abstract
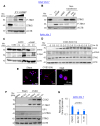
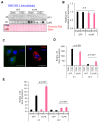
Enteroviruses are a genus of small RNA viruses that are responsible for approximately one billion global infections annually. These infections range in severity from the common cold and flu-like symptoms to more severe diseases, such as viral myocarditis, pancreatitis, and neurological disorders, that continue to pose a global health challenge with limited therapeutic strategies currently available. In the current study, we sought to understand the interaction between coxsackievirus B3 (CVB3), which is a model enterovirus, and macrophage cells, as there is limited understanding of how this virus interacts with macrophage innate immune cells. Our study demonstrated that CVB3 can robustly activate macrophages without apparent viral replication in these cells. We also showed that myeloid cells lacked the viral entry receptor coxsackievirus and adenovirus receptor (CAR). However, the expression of exogenous CAR in RAW264.7 macrophages was unable to overcome the viral replication deficit. Interestingly, the CAR expression was associated with altered inflammatory responses during prolonged infection. Additionally, we identified the autophagy protein LC3 as a novel stimulus for macrophage activation. These findings provide new insights into the mechanisms of CVB3-induced macrophage activation and its implications for viral pathogenesis.
Keywords: LC3; coxsackievirus B3 (CVB3); coxsackievirus and adenovirus receptor (CAR); innate immunity; macrophages.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Mitochondrial injury and complement dysregulation are drivers of pathological inflammation in viral myocarditis.J Virol. 2025 Feb 25;99(2):e0180424. doi: 10.1128/jvi.01804-24. Epub 2025 Jan 23. J Virol. 2025. PMID: 39846741 Free PMC article.
-
A food-responsive switch modulates TFEB and autophagy, and determines susceptibility to coxsackievirus infection and pancreatitis.Autophagy. 2021 Feb;17(2):402-419. doi: 10.1080/15548627.2020.1720425. Epub 2020 Feb 4. Autophagy. 2021. PMID: 32019403 Free PMC article.
-
Coxsackievirus group B3 regulates ASS1-mediated metabolic reprogramming and promotes macrophage inflammatory polarization in viral myocarditis.J Virol. 2024 Sep 17;98(9):e0080524. doi: 10.1128/jvi.00805-24. Epub 2024 Aug 28. J Virol. 2024. PMID: 39194244 Free PMC article.
-
Manipulating intestinal immunity and microflora: an alternative solution to viral myocarditis?Future Microbiol. 2012 Oct;7(10):1207-16. doi: 10.2217/fmb.12.96. Future Microbiol. 2012. PMID: 23030425 Review.
-
CAR: A key regulator of adhesion and inflammation.Int J Biochem Cell Biol. 2017 Aug;89:1-5. doi: 10.1016/j.biocel.2017.05.025. Epub 2017 May 22. Int J Biochem Cell Biol. 2017. PMID: 28545889 Review.
Cited by
-
Human Primary Macrophages Can Transmit Coxsackie B4 Virus to Pancreatic Cells In Vitro.J Med Virol. 2024 Dec;96(12):e70102. doi: 10.1002/jmv.70102. J Med Virol. 2024. PMID: 39614711 Free PMC article. No abstract available.
-
Mitochondrial injury and complement dysregulation are drivers of pathological inflammation in viral myocarditis.J Virol. 2025 Feb 25;99(2):e0180424. doi: 10.1128/jvi.01804-24. Epub 2025 Jan 23. J Virol. 2025. PMID: 39846741 Free PMC article.
References
-
- Newland J.G., Romero J.R. Enteroviruses. In: Heggenhougen H.K., editor. International Encyclopedia of Public Health. Academic Press; Oxford, UK: 2008. pp. 347–352.
-
- Jagdeo J.M., Dufour A., Klein T., Solis N., Kleifeld O., Kizhakkedathu J., Luo H., Overall C.M., Jan E. N-Terminomics TAILS Identifies Host Cell Substrates of Poliovirus and Coxsackievirus B3 3C Proteinases That Modulate Virus Infection. J. Virol. 2018;92:10-1128. doi: 10.1128/JVI.02211-17. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

