Immunogenicity of an Inactivated COVID-19 Vaccine in People Living with HIV in Guangxi, China: A Prospective Cohort Study
- PMID: 39339957
- PMCID: PMC11437430
- DOI: 10.3390/v16091481
Immunogenicity of an Inactivated COVID-19 Vaccine in People Living with HIV in Guangxi, China: A Prospective Cohort Study
Abstract
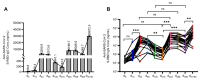
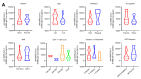
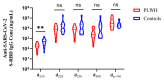
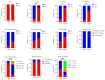
The inactivated COVID-19 vaccine has demonstrated high efficacy in the general population through extensive clinical and real-world studies. However, its effectiveness in immunocompromised individuals, particularly those living with HIV (PLWH), remains limited. In this study, 20 PLWH and 15 HIV-seronegative individuals were recruited to evaluate the immunogenicity of an inactivated COVID-19 vaccine in PLWH through a prospective cohort study. The median age of the 20 PLWH and 15 HIV-seronegative individuals was 42 years and 31 years, respectively. Of the PLWH, nine had been on ART for over five years. The median anti-SARS-CoV-2 S-RBD IgG antibody level on d224 was higher than that on d42 (8188.7 ng/mL vs. 3200.9 ng/mL, P < 0.05). Following COVID-19 infection, the antibody level increased to 29,872.5 ng/mL on dre+90, 12.19 times higher than that on d300. Compared with HIV-seronegative individuals, the antibody level in PLWH was lower on d210 (183.3 ng/mL vs. 509.3 ng/mL, P < 0.01), while there was no difference after d224. The symptoms of COVID-19 infection in PLWH were comparable to those in HIV-seronegative individuals. In this study, the inactivated COVID-19 vaccine demonstrated good immunogenicity in PLWH. The protective benefit of booster vaccinations for PLWH cannot be ignored. Implementing a booster vaccination policy for PLWH is an effective approach to providing better protection against the COVID-19 pandemic.
Keywords: PLWH; SARS-CoV-2; immunogenicity; inactivated COVID-19 vaccine.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Comparing Immune Responses to Inactivated Vaccines against SARS-CoV-2 between People Living with HIV and HIV-Negative Individuals: A Cross-Sectional Study in China.Viruses. 2022 Jan 28;14(2):277. doi: 10.3390/v14020277. Viruses. 2022. PMID: 35215870 Free PMC article.
-
Inactivated SARS-CoV-2 vaccines elicit immunogenicity and T-cell responses in people living with HIV.Int Immunopharmacol. 2022 Jan;102:108383. doi: 10.1016/j.intimp.2021.108383. Epub 2021 Nov 18. Int Immunopharmacol. 2022. PMID: 34824035 Free PMC article. Clinical Trial.
-
Immunogenicity and reactogenicity of SARS-CoV-2 vaccines in people living with HIV in the Netherlands: A nationwide prospective cohort study.PLoS Med. 2022 Oct 27;19(10):e1003979. doi: 10.1371/journal.pmed.1003979. eCollection 2022 Oct. PLoS Med. 2022. PMID: 36301821 Free PMC article.
-
Immunogenicity and safety of SARS-CoV-2 recombinant spike protein vaccine in South African people living with and without HIV-1 infection: A phase 2 randomised trial.J Infect. 2024 Dec;89(6):106285. doi: 10.1016/j.jinf.2024.106285. Epub 2024 Sep 27. J Infect. 2024. PMID: 39343247 Clinical Trial.
-
Overview of SARS-CoV-2 infection in adults living with HIV.Lancet HIV. 2021 May;8(5):e294-e305. doi: 10.1016/S2352-3018(21)00070-9. Lancet HIV. 2021. PMID: 33915101 Free PMC article. Review.
References
-
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. - DOI - PMC - PubMed
-
- WHO WHO Coronavirus (COVID-19) Dushboard. 2023. [(accessed on 12 April 2023)]. Available online: https://covid19.who.int/
-
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., et al. Safety and Efficacy of the ChAdOx1 nCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

