Rotavirus-Specific Maternal Serum Antibodies and Vaccine Responses to RV3-BB Rotavirus Vaccine Administered in a Neonatal or Infant Schedule in Malawi
- PMID: 39339964
- PMCID: PMC11437397
- DOI: 10.3390/v16091488
Rotavirus-Specific Maternal Serum Antibodies and Vaccine Responses to RV3-BB Rotavirus Vaccine Administered in a Neonatal or Infant Schedule in Malawi
Abstract
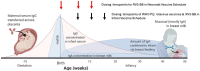
High titres of rotavirus-specific maternal antibodies may contribute to lower rotavirus vaccine efficacy in low- and middle-income countries (LMICs). RV3-BB vaccine (G3P[6]) is based on a neonatal rotavirus strain that replicates well in the newborn gut in the presence of breast milk. This study investigated the association between maternal serum antibodies and vaccine response in infants administered the RV3-BB vaccine. Serum was collected antenatally from mothers of 561 infants enrolled in the RV3-BB Phase II study conducted in Blantyre, Malawi, and analysed for rotavirus-specific serum IgA and IgG antibodies using enzyme-linked immunosorbent assay. Infant vaccine take was defined as cumulative IgA seroconversion (≥3 fold increase) and/or stool vaccine shedding. Maternal IgA or IgG antibody titres did not have a negative impact on vaccine-like stool shedding at any timepoint. Maternal IgG (but not IgA) titres were associated with reduced take post dose 1 (p < 0.005) and 3 (p < 0.05) in the neonatal vaccine schedule group but not at study completion (week 18). In LMICs where high maternal antibodies are associated with low rotavirus vaccine efficacy, RV3-BB in a neonatal or infant vaccine schedule has the potential to provide protection against severe rotavirus disease.
Trial registration: ClinicalTrials.gov NCT03483116.
Keywords: RV3-BB vaccine; maternal antibodies; rotavirus vaccine.
Conflict of interest statement
MCRI holds the patent for the RV3-BB vaccine: J.E.B., E.A.L., B.M., A.H., D.P., N.B.-S., R.B., D.S.O., F.J., and C.M.D. are/were employees of MCRI. C.M.D. has served on advisory boards for GSK (2019, 2021), with all payments directed to an administrative fund held by MCRI. N.C. is a National Institute for Health and Care Research (NIHR) Senior Investigator (NIHR 203756). N.C., A.T., and K.C.J. are affiliated with the NIHR Global Health Research Group on Gastrointestinal Infections at the University of Liverpool. N.C. and K.C.J. are affiliated with the NIHR Health Protection Research Unit in Gastrointestinal Infections at the University of Liverpool, a partnership with the UK Health Security Agency in collaboration with the University of Warwick. Views are those of the authors and not necessarily those of the NIHR, the Department of Health and Social Care, the UK government, or the UK Health Security Agency.
Figures

 Maternal rotavirus-specific serum IgA antibody titres (log).
Maternal rotavirus-specific serum IgA antibody titres (log).  Maternal rotavirus-specific serum IgG antibody titres (log).
Maternal rotavirus-specific serum IgG antibody titres (log).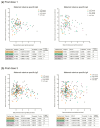
References
-
- International Vaccine Access Centre Vaccine Information Management System (VIMS) Global Rotavirus Vaccine Access Report. 2024. [(accessed on 18 July 2024)]. Available online: https://jhsph.edu/research/centers-and-institutes/ivac/view-hub.org/vacc....
-
- Black R.E., Perin J., Yeung D., Rajeev T., Miller J., Elwood S.E., Platts-Milss J.A. Estimated global and regional causes of deaths from diarrhea in childen younger that 5 years during 2002–21: A systematic review and Bayesian multinomial analysis. Lancet Glob. Health. 2024;12:e919–e928. doi: 10.1016/S2214-109X(24)00078-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

