Validation of Saliva as the Clinical Specimen Type for a University-Wide COVID-19 Surveillance Program
- PMID: 39339970
- PMCID: PMC11437455
- DOI: 10.3390/v16091494
Validation of Saliva as the Clinical Specimen Type for a University-Wide COVID-19 Surveillance Program
Abstract
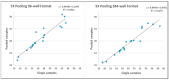
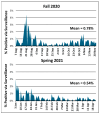
At the beginning of the COVID-19 pandemic, the Georgia Institute of Technology made the decision to keep the university doors open for on-campus attendance. To manage COVID-19 infection rates, internal resources were applied to develop and implement a mass asymptomatic surveillance program. The objective was to identify infections early for proper follow-on verification testing, contact tracing, and quarantine/isolation as needed. Program success depended on frequent and voluntary sample collection from over 40,000 students, faculty, and staff personnel. At that time, the nasopharyngeal (NP) swab, not saliva, was the main accepted sample type for COVID-19 testing. However, due to collection discomfort and the inability to be self-collected, the NP swab was not feasible for voluntary and frequent self-collection. Therefore, saliva was selected as the clinical sample type and validated. A saliva collection kit and a sample processing and analysis workflow were developed. The results of a clinical sample-type comparison study between co-collected and matched NP swabs and saliva samples showed 96.7% positive agreement and 100% negative agreement. During the Fall 2020 and Spring 2021 semesters, 319,988 samples were collected and tested. The program resulted in maintaining a low overall mean positivity rate of 0.78% and 0.54% for the Fall 2020 and Spring 2021 semesters, respectively. For this high-throughput asymptomatic COVID-19 screening application, saliva was an exceptionally good sample type.
Keywords: COVID-19; RNA; SARS-CoV-2; nasopharyngeal swap; saliva; surveillance.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Panovska-Griffiths J., Kerr G.C., Stuart R.M., Mistry D., Klein D.J., Viner R.M., Bonell C. Determining the optimal strategy for reopening schools, the impact of test and trace interventions, and the risk of occurrence of a second COVID-19 epidemic wave in the UK: A modelling study. Lancet Child Adolesc. Health. 2020;4:817–827. doi: 10.1016/S2352-4642(20)30250-9. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

