(Re-)Emergence of Oropouche Virus (OROV) Infections: Systematic Review and Meta-Analysis of Observational Studies
- PMID: 39339974
- PMCID: PMC11437499
- DOI: 10.3390/v16091498
(Re-)Emergence of Oropouche Virus (OROV) Infections: Systematic Review and Meta-Analysis of Observational Studies
Abstract
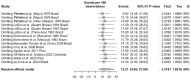
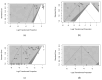
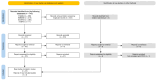
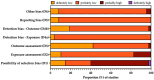
Oropouche Virus (OROV; genus of Orthobunyavirus) is the causal agent of Oropouche Fever (OF). Due to the lack of specific signs and symptoms and the limited availability of diagnostic tests, the actual epidemiology of OROV infections and OF has been extensively disputed. In this systematic review with meta-analysis, a literature search was carried out in PubMed, Scopus, EMBASE, and MedRxiv in order to retrieve relevant articles on the documented occurrence of OROV infections. Pooled detection rates were then calculated for anti-OROV antibodies and virus detection (i.e., viral RNA detected by viral cultures and/or real-time polymerase chain reaction [RT-qPCR]). Where available, detection rates for other arboviruses (i.e., Dengue [DENV], Chikungunya [CHKV], and Zika Virus [ZIKV]) were calculated and compared to those for OROV. A total of 47 studies from South America and the Caribbean were retrieved. In individuals affected by febrile illness during OROV outbreaks, a documented prevalence of 0.45% (95% confidence interval [95%CI] 0.16 to 1.12) for virus isolation, 12.21% (95%CI 4.96 to 27.09) for seroprevalence (including both IgM and IgG class antibodies), and 12.45% (95%CI 3.28 to 37.39) for the detection of OROV-targeting IgM class antibodies were eventually documented. In the general population, seroprevalence was estimated to be 24.45% (95%CI 7.83 to 55.21) for IgG class antibodies. The OROV detection rate from the cerebrospinal fluids of suspected cases of viral encephalitis was estimated to be 2.40% (95%CI 1.17 to 5.03). The occurrence of OROV infections was consistently lower than that of DENV, CHKV, and ZIKV during outbreaks (Risk Ratio [RR] 24.82, 95%CI 21.12 to 29.16; RR 2.207, 95%CI 1.427 to 3.412; and RR 7.900, 95%CI 5.386 to 11.578, respectively) and in the general population (RR 23.614, 95%CI 20.584 to 27.129; RR 3.103, 95%CI 2.056 to 4.685; and RR 49.500, 95%CI 12.256 to 199.921, respectively). In conclusion, our study stresses the possibly high underestimation of OROV prevalence in the general population of South America, the potential global threat represented by this arbovirus infection, and the potential preventive role of a comprehensive "One Health approach".
Keywords: Oropouche Virus; Orthobunyavirus; arbovirus; vector-borne disease.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







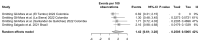







References
-
- Pereira R.S., Colangelo J.F., Souza P.G.A., de Carvalho L.G.F., da Cruz Nizer W.S., Lima W.G. Epidemiological Aspects of the Oropouche Virus (Orthobunyavirus) in South America: A Systematic Review. Rev. Colomb. Cienc. Quim. Farm. 2022;51:166–184. doi: 10.15446/rcciquifa.v51n1.102689. - DOI
-
- Tilston-Lunel N.L., Hughes J., Acrani G.O., da Silva D.E.A., Azevedo R.S.S., Rodrigues S.G., Vasconcelos P.F.C., Nunes M.R.T., Elliott R.M. Genetic Analysis of Members of the Species Oropouche Virus and Identification of a Novel M Segment Sequence. J. Gen. Virol. 2015;96:1636–1650. doi: 10.1099/vir.0.000108. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

