The Clinical Effectiveness of Single-Dose Human Papillomavirus Vaccination
- PMID: 39339988
- PMCID: PMC11436243
- DOI: 10.3390/vaccines12090956
The Clinical Effectiveness of Single-Dose Human Papillomavirus Vaccination
Abstract
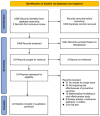
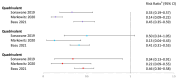
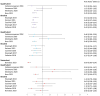
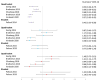
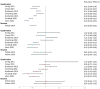
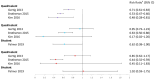
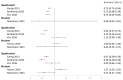
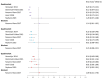
The human papillomavirus (HPV) vaccine was initially approved for a three-dose regimen. Due to resource limitations, budget constraints, low acceptance, and poor adherence, global vaccination coverage is only 15%. A single-dose regimen could simplify logistics, reduce costs, and improve accessibility. However, its clinical effectiveness remains debatable. This review systematically searched PubMed, Embase, and Cochrane Library, including 42 clinical studies, to assess the effectiveness of a single-dose HPV vaccination for preventing HPV infections, cervical abnormalities, and genital warts. We summarized the effectiveness of bivalent, quadrivalent, and nonavalent vaccines across different age groups and buffer periods, and analyzed the factors contributing to the inconsistency of results. The review also provides insights into designing robust future research to inform single-dose HPV vaccination policies and guidelines, highlighting the need for further research to refine vaccination strategies.
Keywords: dose; effectiveness; human papillomavirus; vaccine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

