Immunometabolic Regulation of Vaccine-Induced Antibody Responses in Aging Mice
- PMID: 39339992
- PMCID: PMC11436058
- DOI: 10.3390/vaccines12090960
Immunometabolic Regulation of Vaccine-Induced Antibody Responses in Aging Mice
Abstract
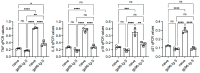
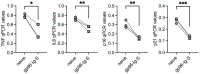
Immune cells undergo metabolic reprogramming to meet the demands associated with immune responses. The effects of aging on these pathways and on the metabolic phenotype of the immune cells participating in antibody responses to vaccines are still largely unknown. Here we used a vaccine for SARS-CoV-2 that utilizes the cellular heat shock chaperone glycoprotein 96 (gp96), engineered to co-express SARS-CoV-2 Spike (spike) protein (gp96-Ig-S). Results show that this vaccine induces comparable B cell primary responses in young and old mice at later time points, but a significantly lesser secondary response in old as compared to young mice, with the antibodies generated in the secondary response being also of lower avidity. This occurs because aging changes the B cell metabolic phenotype and induces hyper-metabolic B cells that are associated with higher intrinsic inflammation and decreased protective antibody responses. However, the gp96-Ig-S vaccine was found to be effective in significantly reducing the metabolic/inflammatory status of B cells from old mice, suggesting the possibility that targeting metabolic pathways may improve immune function in old mice that do not respond adequately to the vaccine.
Keywords: B cells; immunometabolism; inflammation; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

