Enhanced Effects of ISA 207 Adjuvant via Intradermal Route in Foot-and-Mouth Disease Vaccine for Pigs
- PMID: 39339996
- PMCID: PMC11435775
- DOI: 10.3390/vaccines12090963
Enhanced Effects of ISA 207 Adjuvant via Intradermal Route in Foot-and-Mouth Disease Vaccine for Pigs
Abstract
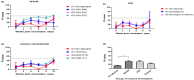
In South Korea, a mandatory nation-wide foot-and-mouth disease (FMD) vaccination policy is in place. However, a major side effect of the current method of intramuscular (IM) administration of oil-adjuvanted FMD vaccines is the formation of granulomas in the muscles of pigs. To address this issue, we assessed the possible application of intradermal (ID) vaccination. Initially, we compared the serological immune response in specific pathogen-free pigs inoculated with FMD vaccines formulated with eight different adjuvants, administered twice at the neck site using a syringe with a needle via the ID route. Among the formulations (water-in-oil-in-water (W/O/W), oil-in-water (O/W), and polymer nanomaterials), ISA 207 of W/O/W was the most effective in inducing immunogenicity followed by ISA 201 of W/O/W. ISA 207 was further tested in formulations of different antigen doses (12 or 1.2 μg) delivered via both IM and ID routes. All four treatments successfully protected the pigs against FMD virus challenges. To assess the feasibility of the field application of the vaccines with ISA 207, we conducted ID vaccination of conventional pigs using a needle-free device, resulting in the detection of significant levels of neutralizing antibodies. ISA 207 was shown to be superior to ISA 201 in inducing immunogenicity via the ID route. In conclusion, ISA 207 could be a suitable adjuvant for ID vaccination in terms of vaccine efficacy for FMD, allowing for alternate use of ID vaccination and subsequent reduction in the incidences of granuloma formation in the field.
Keywords: adjuvant; foot-and-mouth disease; intradermal route; protection; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Montanide ISA™ 201 adjuvanted FMD vaccine induces improved immune responses and protection in cattle.Vaccine. 2013 Jul 18;31(33):3327-32. doi: 10.1016/j.vaccine.2013.05.078. Epub 2013 Jun 2. Vaccine. 2013. PMID: 23735678
-
Needleless intradermal vaccination for foot-and-mouth disease induced granuloma-free effective protection in pigs.J Vet Sci. 2019 May;20(3):e29. doi: 10.4142/jvs.2019.20.e29. J Vet Sci. 2019. PMID: 31161747 Free PMC article.
-
Immunogenicity and Protection against Foot-and-Mouth Disease Virus in Swine Intradermally Vaccinated with a Bivalent Vaccine of Foot-and-Mouth Disease Virus Type O and A.Vaccines (Basel). 2023 Apr 7;11(4):815. doi: 10.3390/vaccines11040815. Vaccines (Basel). 2023. PMID: 37112726 Free PMC article.
-
Enhancing Immunogenicity of Influenza Vaccine in the Elderly through Intradermal Vaccination: A Literature Analysis.Viruses. 2022 Nov 3;14(11):2438. doi: 10.3390/v14112438. Viruses. 2022. PMID: 36366536 Free PMC article. Review.
-
An overview of the immunogenicity and effectiveness of current human rabies vaccines administered by intradermal route.Vaccine. 2019 Oct 3;37 Suppl 1:A99-A106. doi: 10.1016/j.vaccine.2018.11.072. Epub 2018 Dec 11. Vaccine. 2019. PMID: 30551985 Review.
References
-
- Ibrahim Eel S., Gamal W.M., Hassan A.I., Mahdy Sel D., Hegazy A.Z., Abdel-Atty M.M. Comparative study on the immunopotentiator effect of ISA 201, ISA 61, ISA 50, ISA 206 used in trivalent foot and mouth disease vaccine. Vet. World. 2015;8:1189–1198. doi: 10.14202/vetworld.2015.1189-1198. - DOI - PMC - PubMed
-
- Saravanan S., Umapathi V., Priyanka M., Hosamani M., Sreenivasa B.P., Patel B.H.M., Narayanan K., Sanyal A., Basagoudanavar S.H. Hematological and serum biochemical profile in cattle experimentally infected with foot-and-mouth disease virus. Vet. World. 2020;13:426–432. doi: 10.14202/vetworld.2020.426-432. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

