Effects of Rotavirus NSP4 on the Immune Response and Protection of Rotavirus-Norovirus Recombinant Subunit Vaccines in Different Immune Pathways
- PMID: 39340055
- PMCID: PMC11436106
- DOI: 10.3390/vaccines12091025
Effects of Rotavirus NSP4 on the Immune Response and Protection of Rotavirus-Norovirus Recombinant Subunit Vaccines in Different Immune Pathways
Abstract
Diarrheal disease continues to be a major cause of global morbidity and mortality among children under 5 years of age. To address the current issues associated with oral attenuated rotavirus vaccines, the study of parenteral rotavirus vaccines has promising prospects. In our previous study, we reported that rotavirus nonstructural protein 4 (NSP4) did not increase the IgG antibody titer of co-immune antigen but did have a protective effect against diarrhea via the intramuscular injection method. Here, we explored whether NSP4 can exert adjuvant effects on mucosal immune pathways. In this study, we immunized mice via muscle and nasal routes, gavaged them with the rotavirus Wa strain or the rotavirus SA11 strain, and then tested the protective effects of immune sera against both viruses. The results revealed that the serum-specific VP8* IgG antibody titers of the mice immunized via the nasal route were much lower than those of the mice immunized by intramuscular injection, and the specific IgA antibodies were almost undetectable in the bronchoalveolar lavage fluid (BALF). NSP4 did not increase the titer of specific VP8* antibodies in either immune pathway. Therefore, in the two vaccines (PP-NSP4-VP8* and PP-VP8*+NSP4) used in this study, NSP4 was unable to perform its potential adjuvant role through the mucosal immune pathway. Instead, NSP4 was used as a co-immunized antigen to stimulate the mice to produce specific binding antibodies that play a protective role against diarrhea.
Keywords: immune method; nonstructural protein 4; norovirus P particle; recombinant subunit vaccine; rotavirus.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
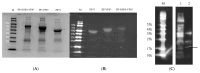

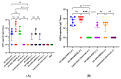
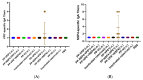
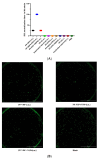
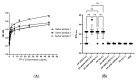
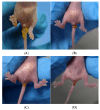
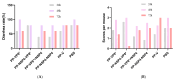

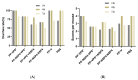

References
Grants and funding
- 2022YFC2305700/the National Key R&D Program of China
- 82104130/the National Natural Science Foundation of China
- 202205AC160015/Funds for High-Level Scientific and Technological Talents Selection Special Project of Yunnan Province
- 202002AA100009/the Major Science and Technology Special Projects of Yunnan Province
- 202401AT070148, 202201AU070150, and 202301AT070362/Yunnan Fundamental Research Projects
LinkOut - more resources
Full Text Sources
Miscellaneous

