The Effectiveness and Sero-Immunity of Hepatitis B Vaccination in People Who Use Drugs: A Systematic Review and Meta-Analysis
- PMID: 39340056
- PMCID: PMC11435961
- DOI: 10.3390/vaccines12091026
The Effectiveness and Sero-Immunity of Hepatitis B Vaccination in People Who Use Drugs: A Systematic Review and Meta-Analysis
Abstract
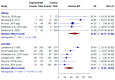
Hepatitis B virus (HBV) vaccination has been available for over four decades. However, a synthesis of the evidence regarding the effectiveness of this strategy on preventing hepatitis B infection in people who use drugs (PWUD) is lacking. A systematic search of the MEDLINE (via PubMed), SCOPUS, Web of Science, and Cochrane Library databases was conducted up to June 2024. Eight studies reported on the effectiveness of HBV vaccination, defined as a positive result for HBsAg or anti-Hbc in vaccinated versus non-vaccinated PWUD, with a pooled effect size of 52% (95% CI: 28.2-67.9) for HBsAg and 31.89% (95% CI: 14.8-45.5) for anti-Hbc. For sero-immunity, defined as the proportion of vaccinated PWUD with levels of anti-HBs ≥ 10 mIU/mL, we found that 66.2% (95% CI: 0.61-0.71; I2 = 94%) had protective levels of anti-HBs. The results of this meta-analysis indicate that the incidence of HBV infection is lower in vaccinated PWUD than in those who did not receive the vaccine. However, the effectiveness is lower than that observed in the general population. This highlights the need for a thorough review of the factors influencing the prevention of HBV infection in PWUD.
Keywords: drug users; hepatitis b virus; immunity; immunization; meta-analysis; systematic review.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Tripathi N., Mousa O.Y. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2024. Hepatitis B. - PubMed
-
- Sheena B.S., Hiebert L., Han H., Ippolito H., Abbasi-Kangevari M., Abbasi-Kangevari Z., Abbastabar H., Abdoli A., Abubaker Ali H., Adane M.M., et al. Global, Regional, and National Burden of Hepatitis B, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 2022;7:796–829. doi: 10.1016/S2468-1253(22)00124-8. - DOI - PMC - PubMed
-
- European Centre for Disease Prevention and Control . Prevention of Hepatitis B and C in the EU/EEA. European Centre for Disease Prevention and Control; Stockholm, Sweden: 2022.
Publication types
LinkOut - more resources
Full Text Sources