The Improvement of Adaptive Immune Responses towards COVID-19 Following Diphtheria-Tetanus-Pertussis and SARS-CoV-2 Vaccinations in Indonesian Children: Exploring the Roles of Heterologous Immunity
- PMID: 39340062
- PMCID: PMC11435621
- DOI: 10.3390/vaccines12091032
The Improvement of Adaptive Immune Responses towards COVID-19 Following Diphtheria-Tetanus-Pertussis and SARS-CoV-2 Vaccinations in Indonesian Children: Exploring the Roles of Heterologous Immunity
Abstract
Background: Routine childhood vaccination, e.g., for diphtheria, tetanus, and pertussis (DTP), might provide additional protection against SARS-CoV-2 infection. This concept of heterologous immunity was explored in healthy children receiving both DTP and inactivated SARS-CoV-2 vaccines.
Methods: A cross-sectional study was performed on 154 healthy children aged 6-8 years old in Jakarta, Indonesia. Their vaccination status for the DTP (including a diphtheria-tetanus booster vaccine at 5 years old) and CoronaVac (from 6 years old) vaccines were recorded. Peripheral blood samples were collected from all participants, in which anti-diphtheria toxoid IgG and anti-SARS-CoV-2 S-RBD antibodies and T cell-derived IFN-γ were measured.
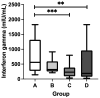
Results: The study participants with complete DTP vaccination had significantly higher titers of anti-diphtheria toxoid IgG than the ones without (median = 0.9349 versus 0.2113 IU/mL; p < 0.0001). Upon stratification based on DTP and CoronaVac vaccination statuses, the participants with complete DTP and CoronaVac vaccinations had the highest titer of anti-SARS-CoV-2 S-RBD antibodies (median = 1196 U/mL) and the highest concentration of SARS-CoV-2-specific T cell-derived IFN-γ (median = 560.9 mIU/mL) among all the groups.
Conclusions: Healthy children aged 6-8 years old with complete DTP and CoronaVac vaccinations exhibited stronger SARS-CoV-2-specific T cell immune responses. This might suggest an additional benefit of routine childhood vaccination in generating protection against novel pathogens, presumably via heterologous immunity.
Keywords: SARS-CoV-2-specific immune response; children; diphtheria–tetanus–pertussis vaccine; heterologous immunity; inactivated COVID-19 vaccine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Safety and immunogenicity of combined diphtheria-tetanus-pertussis (whole cell and acellular)-Haemophilus influenzae-b conjugate vaccines administered to Indonesian children.Vaccine. 1999 Mar 17;17(11-12):1384-93. doi: 10.1016/s0264-410x(98)00402-2. Vaccine. 1999. PMID: 10195774 Clinical Trial.
-
Potential Cross-Reactive Immunity to SARS-CoV-2 From Common Human Pathogens and Vaccines.Front Immunol. 2020 Oct 16;11:586984. doi: 10.3389/fimmu.2020.586984. eCollection 2020. Front Immunol. 2020. PMID: 33178220 Free PMC article.
-
Seroimmunity to diphtheria and tetanus among mother-infant pairs; the role of maternal immunity on infant immune response to diphtheria-tetanus vaccination.Swiss Med Wkly. 2008 May 3;138(17-18):256-60. doi: 10.4414/smw.2008.11840. Swiss Med Wkly. 2008. PMID: 18481231
-
Diphtheria-tetanus-pertussis vaccine: past, current & future.Future Microbiol. 2022 Feb;17:185-197. doi: 10.2217/fmb-2021-0167. Epub 2021 Dec 3. Future Microbiol. 2022. PMID: 34856810 Review.
-
Waning Immunity After Receipt of Pertussis, Diphtheria, Tetanus, and Polio-Related Vaccines: A Systematic Review and Meta-analysis.J Infect Dis. 2022 Feb 15;225(4):557-566. doi: 10.1093/infdis/jiab480. J Infect Dis. 2022. PMID: 34543411
References
-
- World Health Organization WHO COVID-19 Dashboard. [(accessed on 22 June 2024)]. Available online: https://data.who.int/dashboards/covid19/cases.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

