Dynamic Vaccine Allocation for Control of Human-Transmissible Disease
- PMID: 39340064
- PMCID: PMC11435756
- DOI: 10.3390/vaccines12091034
Dynamic Vaccine Allocation for Control of Human-Transmissible Disease
Abstract
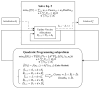
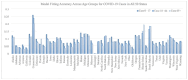
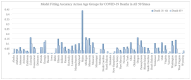
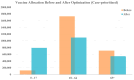
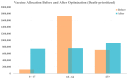
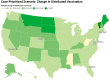
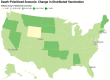
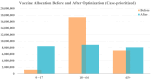
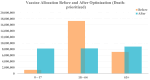
During pandemics, such as COVID-19, supplies of vaccines can be insufficient for meeting all needs, particularly when vaccines first become available. Our study develops a dynamic methodology for vaccine allocation, segmented by region, age, and timeframe, using a time-sensitive, age-structured compartmental model. Based on the objective of minimizing a weighted sum of deaths and cases, we used the Sequential Least Squares Quadratic Programming method to search for a locally optimal COVID-19 vaccine allocation for the United States, for the period from 16 December 2020 to 30 June 2021, where regions corresponded to the 50 states in the United States (U.S.). We also compared our solution to actual allocations of vaccines. From our model, we estimate that approximately 1.8 million cases and 9 thousand deaths could have been averted in the U.S. with an improved allocation. When case reduction is prioritized over death reduction, we found that young people (17 and younger) should receive priority over old people due to their potential to expose others. However, if death reduction is prioritized over case reduction, we found that more vaccines should be allocated to older people, due to their propensity for severe disease. While we have applied our methodology to COVID-19, our approach generalizes to other human-transmissible diseases, with potential application to future epidemics.
Keywords: COVID-19; transmission modeling; vaccine allocation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Self W.H., Tenforde M.W., Rhoads J.P., Gaglani M., Ginde A.A., Douin D.J., Olson S.M., Talbot H.K., Casey J.D., Mohr N.M., et al. Comparative effectiveness of Moderna, Pfizer-Biontech, and Janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions—united states, march–august 2021. Morb. Mortal. Wkly. Rep. 2021;70:1337. - PMC - PubMed
-
- Schimit P.H.T., Monteiro L.H.A. A Vaccination Game Based on Public Health Actions and Personal Decisions. Ecol. Model. 2011;222:1651–1655. doi: 10.1016/j.ecolmodel.2011.02.019. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

