Safety and Immunogenicity of a Carbohydrate Fatty Acid Monosulphate Ester Adjuvant Combined with a Low-Dose Quadrivalent Split-Virion Inactivated Influenza Vaccine: A Randomised, Observer-Blind, Active-Controlled, First-in-Human, Phase 1 Study
- PMID: 39340066
- PMCID: PMC11435821
- DOI: 10.3390/vaccines12091036
Safety and Immunogenicity of a Carbohydrate Fatty Acid Monosulphate Ester Adjuvant Combined with a Low-Dose Quadrivalent Split-Virion Inactivated Influenza Vaccine: A Randomised, Observer-Blind, Active-Controlled, First-in-Human, Phase 1 Study
Abstract
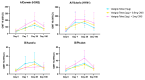
Seasonal influenza vaccine effectiveness is low. Carbohydrate fatty acid monosulphate ester (CMS), a new oil-in-water adjuvant, has proven potency in animal models with suggested capacity for dose-sparing. The objective was to evaluate safety and immunogenicity of CMS when added to a low-dose influenza vaccine (QIV) in humans. In a randomised, double-blind, active-controlled, first-in-human study, sixty participants (18-50 years) received either 0.5 mg CMS or 2 mg CMS with 1/5th dose QIV, or a full dose QIV without CMS. Adverse events (AE) were monitored until 7 days post-vaccination. Haemagglutinin inhibition (HI) titres in serum and CD4+ T cells in PBMCs were determined at day 0, 7, 28, and 180. Mean age was 37.6 (±10.1) years and 42/60 (70.0%) were female. Pain at injection site (42/60, 86.7%) and headache (34/60, 56.7%) were reported most and more frequently in the 2 mg CMS group. HI titres and the frequency of influenza specific CD4+ T cells were equal across strains for the three cohorts on all visits, increased until day 28 and decreased at day 180 to values higher than baseline. CMS was safe in humans. Humoral and cell-mediated immunogenicity was similar across vaccines, even with 1/5th antigen dose. CMS can have beneficial implications in low-resource settings or in a pandemic context.
Keywords: adjuvant; dose-sparing; immunogenicity; influenza vaccine; safety.
Conflict of interest statement
E.M. is an employee of Harmony Clinical Research BV and F.V. and A.M. are employees of VisMederi S.r.l. G.L.-R. provided consulting services and received consulting fees from Virometix, Osivax, ICON Genetics, Sumitomo Pharma, Curevo, and Minervax. P.P.P. and L.H. are co-inventors of a patent related to CMS as vaccine adjuvant. All IP rights are assigned to LiteVax BV, and P.P.P and L.H. hold shares in LiteVax BV. I.L.-R. declares that her institution received funding from GSK, Icosavax, Virometix, Janssen Vaccines, Curevac, Moderna, Osivax, MSD, ICON Genetics, and OSE Immunotherapeutics for other vaccine trials; and from Janssen Vaccines and MSD for consulting services. All others declare no conflicts of interest.
Figures



References
-
- World Health Organization (WHO) Influenza (Seasonal) Factsheet. [(accessed on 19 December 2023)]. Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal)
-
- Carregaro R.L., Roscani A.N.C.P., Raimundo A.C.S., Ferreira L., Vanni T., da Graça Salomão M., Probst L.F., Viscondi J.Y.K. Immunogenicity and safety of inactivated quadrivalent influenza vaccine compared with the trivalent vaccine for influenza infection: An overview of systematic reviews. BMC Infect. Dis. 2023;23:563. doi: 10.1186/s12879-023-08541-0. - DOI - PMC - PubMed
-
- ECDC Factsheet about Seasonal Influenza. [(accessed on 14 May 2020)]. Available online: https://www.ecdc.europa.eu/en/seasonal-influenza/facts/factsheet.
-
- Iuliano A.D., Roguski K.M., Chang H.H., Muscatello D.J., Palekar R., Tempia S., Cohen C., Gran J.M., Schanzer D., Cowling B.J., et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet. 2018;391:1285–1300. doi: 10.1016/S0140-6736(17)33293-2. - DOI - PMC - PubMed
-
- Erbelding E.J., Post D.J., Stemmy E.J., Roberts P.C., Augustine A.D., Ferguson S., Paules C.I., Graham B.S., Fauci A.S. A Universal Influenza Vaccine: The Strategic Plan for the National Institute of Allergy and Infectious Diseases. J. Infect. Dis. 2018;218:347–354. doi: 10.1093/infdis/jiy103. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

