Evaluating Nanoparticulate Vaccine Formulations for Effective Antigen Presentation and T-Cell Proliferation Using an In Vitro Overlay Assay
- PMID: 39340079
- PMCID: PMC11435973
- DOI: 10.3390/vaccines12091049
Evaluating Nanoparticulate Vaccine Formulations for Effective Antigen Presentation and T-Cell Proliferation Using an In Vitro Overlay Assay
Abstract
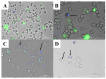
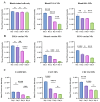
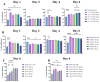
Inducing T lymphocyte (T-cell) activation and proliferation with specificity against a pathogen is crucial in vaccine formulation. Assessing vaccine candidates' ability to induce T-cell proliferation helps optimize formulation for its safety, immunogenicity, and efficacy. Our in-house vaccine candidates use microparticles (MPs) and nanoparticles (NPs) to enhance antigen stability and target delivery to antigen-presenting cells (APCs), providing improved immunogenicity. Typically, vaccine formulations are screened for safety and immunostimulatory effects using in vitro methods, but extensive animal testing is often required to assess immunogenic responses. We identified the need for a rapid, intermediate screening process to select promising candidates before advancing to expensive and time-consuming in vivo evaluations. In this study, an in vitro overlay assay system was demonstrated as an effective high-throughput preclinical testing method to evaluate the immunogenic properties of early-stage vaccine formulations. The overlay assay's effectiveness in testing particulate vaccine candidates for immunogenic responses has been evaluated by optimizing the carboxyfluorescein succinimidyl ester (CFSE) T-cell proliferation assay. DCs were overlaid with T-cells, allowing vaccine-stimulated DCs to present antigens to CFSE-stained T-cells. T-cell proliferation was quantified using flow cytometry on days 0, 1, 2, 4, and 6 upon successful antigen presentation. The assay was tested with nanoparticulate vaccine formulations targeting Neisseria gonorrhoeae (CDC F62, FA19, FA1090), measles, H1N1 flu prototype, canine coronavirus, and Zika, with adjuvants including Alhydrogel® (Alum) and AddaVax™. The assay revealed robust T-cell proliferation in the vaccine treatment groups, with variations between bacterial and viral vaccine candidates. A dose-dependent study indicated immune stimulation varied with antigen dose. These findings highlight the assay's potential to differentiate and quantify effective antigen presentation, providing valuable insights for developing and optimizing vaccine formulations.
Keywords: CFSE; T-cell proliferation; antigen presentation; antigen-presenting cell; cell-to-cell contact; dendritic cell; in vitro vaccine screening; overlay assay.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of this study, in the collection, analysis, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Dhawan M., Rabaan A.A., Fawarah M.M.A., Almuthree S.A., Alsubki R.A., Alfaraj A.H., Mashraqi M.M., Alshamrani S.A., Abduljabbar W.A., Alwashmi A.S.S., et al. Updated Insights into the T Cell-Mediated Immune Response against SARS-CoV-2: A Step towards Efficient and Reliable Vaccines. Vaccines. 2023;11:101. doi: 10.3390/vaccines11010101. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

