A Serum Multi-Parametric Analysis Identifies an Early Innate Immune Signature Associated to Increased Vaccine-Specific Antibody Production and Seroconversion in Simultaneous COVID-19 mRNA and Cell-Based Quadrivalent Influenza Vaccination
- PMID: 39340080
- PMCID: PMC11436141
- DOI: 10.3390/vaccines12091050
A Serum Multi-Parametric Analysis Identifies an Early Innate Immune Signature Associated to Increased Vaccine-Specific Antibody Production and Seroconversion in Simultaneous COVID-19 mRNA and Cell-Based Quadrivalent Influenza Vaccination
Abstract
In this pilot study, a multi-parametric analysis comparing immune responses in sera of adult healthy subjects (HS) or people with type 2 diabetes mellitus (T2D) undergoing the single or simultaneous administration of mRNA-based COVID-19 and cellular quadrivalent inactivated influenza vaccines was conducted. While SARS-CoV-2 antibodies remains comparable, influenza antibody titers and seroconversion were significantly higher upon simultaneous vaccination. Magnitude of anti-influenza humoral response closely correlated with an early innate immune signature, previously described for the COVID-19 vaccine, composed of IL-15, IL-6, TNF-α, IFN-γ, CXCL-10 and here extended also to acute-phase protein Pentraxin 3. People with T2D receiving simultaneous vaccination showed a protective response comparable to HS correlating with the early induction of IFN-γ/CXCL10 and a significant reduction of the circulating glucose level due to increased oxidation of glucose digestion and consumption. These data, although preliminary and in-need of validation in larger cohorts, might be exploited to optimize future vaccination in people with chronic disorders, including diabetes.
Keywords: COVID-19; influenza virus; innate immunity; metabolism; vaccination.
Conflict of interest statement
Marco Iannetta reports research funding (to the Institution) from Gilead, GSK and BD Biosciences for unrelated work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
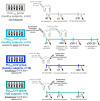
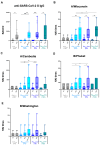
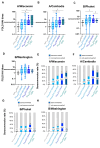
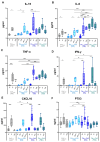

References
-
- CDC Factsheet about Seasonal Influenza. [(accessed on 28 August 2024)]. Available online: https://www.ecdc.europa.eu/en/seasonal-influenza/facts/factsheet.
-
- Lazarus R., Baos S., Cappel-Porter H., Carson-Stevens A., Clout M., Culliford L., Emmett S.R., Garstang J., Gbadamoshi L., Hallis B., et al. Safety and immunogenicity of concomitant administration of COVID-19 vaccines (ChAdOx1 or BNT162b2) with seasonal influenza vaccines in adults in the UK (ComFluCOV): A multicentre, randomised, controlled, phase 4 trial. Lancet. 2021;398:2277–2287. doi: 10.1016/S0140-6736(21)02329-1. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

