Anti-Tumor Immunity to Patient-Derived Breast Cancer Cells by Vaccination with Interferon-Alpha-Conditioned Dendritic Cells (IFN-DC)
- PMID: 39340087
- PMCID: PMC11435915
- DOI: 10.3390/vaccines12091058
Anti-Tumor Immunity to Patient-Derived Breast Cancer Cells by Vaccination with Interferon-Alpha-Conditioned Dendritic Cells (IFN-DC)
Abstract
Background: Breast cancer represents one of the leading causes of death among women. Surgery can be effective, but once breast cancer has metastasized, it becomes extremely difficult to treat. Conventional therapies are associated with substantial toxicity and poor efficacy due to tumor heterogeneity, treatment resistance and disease relapse. Moreover, immune checkpoint blockade appears to offer limited benefit in breast cancer. The poor tumor immunogenicity and the immunosuppressive tumor microenvironment result in scarce T-cell infiltration, leading to a low response rate. Thus, there is considerable interest in the development of improved active immunotherapies capable of sensitizing a patient's immune system against tumor cells.
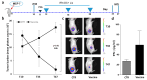
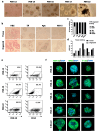
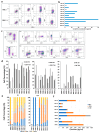
Methods: We evaluated the in vitro anti-tumor activity of a personalized vaccine based on dendritic cells generated in the presence of interferon (IFN)-α and granulocyte-macrophage colony-stimulating factor (IFN-DC) and loaded with an oxidized lysate from autologous tumor cells expanded as 3D organoid culture maintaining faithful tumor antigenic profiles.
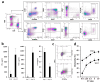
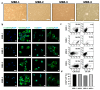
Results: Our findings demonstrate that stimulation of breast cancer patients' lymphocytes with autologous IFN-DC led to efficient Th1-biased response and the generation in vitro of potent cytotoxic activity toward the patients' own tumor cells.
Conclusions: This approach can be potentially applied in association with checkpoint blockade and chemotherapy in the design of new combinatorial therapies for breast cancer.
Keywords: breast cancer; cancer vaccines; dendritic cells; immunotherapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Yang J., Ju J., Guo L., Ji B., Shi S., Yang Z., Gao S., Yuan X., Tian G., Liang Y., et al. Prediction of HER2-positive breast cancer recurrence and metastasis risk from histopathological images and clinical information via multimodal deep learning. Comput. Struct. Biotechnol. J. 2022;20:333–342. doi: 10.1016/j.csbj.2021.12.028. - DOI - PMC - PubMed
-
- Locy H., Verhulst S., Cools W., Waelput W., Brock S., Cras L., Schiettecatte A., Jonckheere J., van Grunsven L.A., Vanhoeij M., et al. Assessing Tumor-Infiltrating Lymphocytes in Breast Cancer: A Proposal for Combining Immunohistochemistry and Gene Expression Analysis to Refine Scoring. Front. Immunol. 2022;13:794175. doi: 10.3389/fimmu.2022.794175. - DOI - PMC - PubMed
-
- Garaud S., Buisseret L., Solinas C., Gu-Trantien C., De Wind A., Van Den Eynden G., Naveaux C., Lodewyckx J.N., Boisson A., Duvillier H., et al. Tumor-infiltrating B cells signal functional humoral immune responses in breast cancer. JCI Insight. 2019;4:e129641. doi: 10.1172/jci.insight.129641. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

