The Evolution of Vaccines Development across Salmonella Serovars among Animal Hosts: A Systematic Review
- PMID: 39340097
- PMCID: PMC11435802
- DOI: 10.3390/vaccines12091067
The Evolution of Vaccines Development across Salmonella Serovars among Animal Hosts: A Systematic Review
Abstract
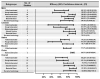
Salmonella is a significant zoonotic foodborne pathogen, and the global spread of multidrug-resistant (MDR) strains poses substantial challenges, necessitating alternatives to antibiotics. Among these alternatives, vaccines protect the community against infectious diseases effectively. This review aims to summarize the efficacy of developed Salmonella vaccines evaluated in various animal hosts and highlight key transitions for future vaccine studies. A total of 3221 studies retrieved from Web of Science, Google Scholar, and PubMed/Medline databases between 1970 and 2023 were evaluated. One hundred twenty-seven qualified studies discussed the vaccine efficacy against typhoidal and nontyphoidal serovars, including live-attenuated vaccines, killed inactivated vaccines, outer membrane vesicles, outer membrane complexes, conjugate vaccines, subunit vaccines, and the reverse vaccinology approach in different animal hosts. The most efficacious vaccine antigen candidate found was recombinant heat shock protein (rHsp60) with an incomplete Freund's adjuvant evaluated in a murine model. Overall, bacterial ghost vaccine candidates demonstrated the highest efficacy at 91.25% (95% CI = 83.69-96.67), followed by the reverse vaccinology approach at 83.46% (95% CI = 68.21-94.1) across animal hosts. More than 70% of vaccine studies showed significant production of immune responses, including humoral and cellular, against Salmonella infection. Collectively, the use of innovative methods rather than traditional approaches for the development of new effective vaccines is crucial and warrants in-depth studies.
Keywords: Salmonellosis; bacterial vaccines; conventional vaccine technologies; immunotherapy; infectious diseases; reverse vaccinology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Frost I., Sati H., Garcia-Vello P., Hasso-Agopsowicz M., Lienhardt C., Gigante V., Beyer P. The role of bacterial vaccines in the fight against antimicrobial resistance: An analysis of the preclinical and clinical development pipeline. Lancet Microbe. 2023;4:e113–e125. doi: 10.1016/S2666-5247(22)00303-2. - DOI - PMC - PubMed
-
- Uzzau S., Marogna G., Leori G.S., Curtiss R., III, Schianchi G., Stocker B.A., Rubino S. Virulence attenuation and live vaccine potential of aroA, crp cdt cya, and plasmid-cured mutants of Salmonella enterica serovar Abortusovis in mice and sheep. Infect. Immun. 2005;73:4302–4308. doi: 10.1128/IAI.73.7.4302-4308.2005. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

