Immune Response after Vaccination against Tick-Borne Encephalitis Virus (TBEV) in Horses
- PMID: 39340104
- PMCID: PMC11435670
- DOI: 10.3390/vaccines12091074
Immune Response after Vaccination against Tick-Borne Encephalitis Virus (TBEV) in Horses
Abstract
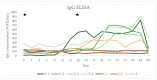
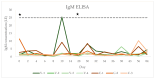
(1) Background: Horses infected by a tick-borne encephalitis virus (TBEV) can develop clinically apparent infections. In humans, vaccination is the most effective preventive measure, while a vaccine is not available for horses. The objective of this study was to describe the immune response in horses after a TBEV vaccination with a human vaccine. (2) Materials and Methods: Seven healthy horses were randomised to a treatment or a control group in a stratified fashion based on TBEV-IgG concentrations on day -4. The treatment group (n = 4) was intramuscularly vaccinated using an inactivated human TBEV vaccine on days 0 and 28; the control group (n = 3) did not receive an injection. A clinical examination and blood sampling were performed on day -4, 0, 2, 4, 6, 8, 10, 14, 28, 30, 32, 34, 36, 38, 43, 56, 84, and 373. A linear mixed model analysis was used to compare IgG and IgM concentrations, neutralising antibody (nAb) titres, leucocyte count, serum amyloid A (SAA), and fibrinogen and globulin concentrations between the groups and time points. (3) Results: The clinical examination was normal in all horses at all time points. There were no significant changes in SAA, globulin, and fibrinogen concentrations and leucocyte count between the groups or time points (all p > 0.05). There was no significant increase in IgG, IgM, or nAb titres in the control group over time (all p > 0.05). In the vaccination group, there was a significant increase in IgG concentration and nAb titres after the second vaccination (p < 0.0001). There was no significant increase in IgM antibodies after the TBEV vaccination (all p > 0.05). One horse in the vaccination group had an IgM concentration above the laboratory reference on day 10. (4) Conclusions: The human TBEV vaccine did not have side effects when used in healthy horses in this study. A significant rise in TBEV-specific IgG antibodies and nAbs after the second vaccination was observed. However, IgG and nAb titres have been shown to decrease within 1 year after vaccination. The results of this study indicate that a vaccination with a human vaccine only induces a mild rise in IgM antibodies and only in previously naive horses. With no significant changes to inflammatory parameters in the vaccinated horses, it remains unclear whether vaccination with the human vaccine leads to protective immunity.
Keywords: IgG; IgM; SAA; TBEV; TBEV vaccination; equine; immune response; neutralising antibodies.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

