SMS-Based Active Surveillance of Adverse Events following Immunization in Children: The VigiVax Study
- PMID: 39340106
- PMCID: PMC11435886
- DOI: 10.3390/vaccines12091076
SMS-Based Active Surveillance of Adverse Events following Immunization in Children: The VigiVax Study
Abstract
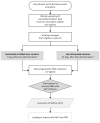
Underreporting is the main limitation of spontaneous reporting systems. This cohort-event monitoring study aims to examine the potential of short message service (SMS)-based surveillance compared to traditional surveillance systems. Using VigiVax software, parents of vaccinated children aged two years or younger, in the period March 2021-May 2022, received a single SMS inquiry about adverse events following immunization (AEFI). Responses were collected, validated by health operators and integrated with the information on electronic immunization registries. AEFI reports were automatically submitted to the Italian Pharmacovigilance system. Among 254,160 SMS messages sent, corresponding to 451,656 administered doses (AD), 71,643 responses were collected (28.2% response rate), and 21,231 of them (8.3%) reported AEFI. After a seriousness assessment based on clinical criteria, 50 reports (0.24%) were classified as serious. Among these, a causality assessment identified 31 reports at least potentially related to the vaccination (RR: 6.86/100,000 AD). Febrile seizures following MMRV (measles, mumps, rubella, varicella) vaccination accounted for 11 of these 31 cases, with an incidence of 32 per 100,000 AD. No fatal outcomes were reported. Our findings support the highly favorable risk profile of pediatric vaccinations and the possibility to improve spontaneous reporting through the integration of digital technologies.
Keywords: active vaccine safety surveillance; adverse event following immunization; digital innovation for vaccine safety surveillance; digital technologies; immunization registries; patient reporting; pediatric vaccination; serious adverse events; short message services.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- EU Parliament . Regulation (EU) No 1235/2010 of the European Parliament and of the Council of 15 December 2010. Official Journal of the European Union; Luxembourg: 2010.
Grants and funding
LinkOut - more resources
Full Text Sources