Neurocardiology: translational advancements and potential
- PMID: 39340173
- PMCID: PMC11955874
- DOI: 10.1113/JP284740
Neurocardiology: translational advancements and potential
Abstract
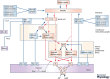
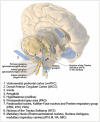
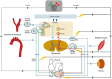
In our original white paper published in the The Journal of Physiology in 2016, we set out our knowledge of the structural and functional organization of cardiac autonomic control, how it remodels during disease, and approaches to exploit such knowledge for autonomic regulation therapy. The aim of this update is to build on this original blueprint, highlighting the significant progress which has been made in the field since and major challenges and opportunities that exist with regard to translation. Imbalances in autonomic responses, while beneficial in the short term, ultimately contribute to the evolution of cardiac pathology. As our understanding emerges of where and how to target in terms of actuators (including the heart and intracardiac nervous system (ICNS), stellate ganglia, dorsal root ganglia (DRG), vagus nerve, brainstem, and even higher centres), there is also a need to develop sensor technology to respond to appropriate biomarkers (electrophysiological, mechanical, and molecular) such that closed-loop autonomic regulation therapies can evolve. The goal is to work with endogenous control systems, rather than in opposition to them, to improve outcomes.
Keywords: autonomic nervous system; cardiac function; parasympathetic; sympathetic nervous system.
© 2024 The Author(s). The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
None declared.
Figures
References
-
- Abboud, H. , Berroir, S. , Labreuche, J. , Orjuela, K. , Amarenco, P. , & Investigators, G. (2006). Insular involvement in brain infarction increases risk for cardiac arrhythmia and death. Annals of Neurology, 59(4), 691–699. - PubMed
-
- Accorsi‐Mendonca, D. , & Machado, B. H. (2013). Synaptic transmission of baro‐ and chemoreceptors afferents in the NTS second order neurons. Autonomic Neuroscience, 175(1–2), 3–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
