Lymph node ratio as an indicator of nodal status in the assessment of survival and recurrence in vulvar cancer: A cohort study
- PMID: 39340307
- PMCID: PMC11439166
- DOI: 10.1177/17455057241285396
Lymph node ratio as an indicator of nodal status in the assessment of survival and recurrence in vulvar cancer: A cohort study
Abstract
Background: Inguinal lymph node (LN) metastasis and particularly the number of metastatic lymph nodes (NMLN) represent a determinant prognostic factor in vulvar squamous cell carcinoma (VSCC). However, the NMLN may be related to the number of removed LNs. Therefore, the lymph node ratio (LNR) reflects not only the burden of LN involvement but also the quality and extent of lymphadenectomy.
Objectives: To investigate the value of the LNR and the count of LN on overall survival (OS) and recurrence-free survival (RFS).
Design: This study is a retrospective, longitudinal, institution-based study.
Methods: This study included 192 patients treated for VSCC at the Salah Azaiez Institute between 1994 and 2022. Clinical, pathological, and evolutionary data were reported. Survival curves were generated by the Kaplan-Meier method, and predictive factors of outcome were analyzed using Cox proportional hazards models.
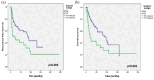
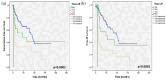
Results: Surgery consisted of a radical vulvectomy, hemivulvectomy, and pelvic exenteration in, respectively, 96.4%, 2.1%, and 1.6% of cases followed by adjuvant radiotherapy in 38.5% of cases. LN dissection was bilateral in 88.5% of cases. LNR = 0, LNR = 0-0.2, and LNR ⩾0.2 were recorded in, respectively, 64.7%, 22.1%, and 13.2% of cases. With a mean follow-up time of 35 ± 42.06 months, the 5-year OS was 52.5% and the 5-year RFS was 55.8%. On multivariate analysis, the independent prognostic factors of OS were the LNR (hazard ratio (HR) = 5.702; 95% confidence interval (CI) = 2.282-14.245; p < 0.0001), Federation of Gynecology and Obstetrics (FIGO) stage (HR = 2.089; 95% CI = 1.028-4.277; p = 0.042), and free margins (HR = 2.247; 95% CI = 1.215-4.155; p = 0.01). Recurrences were recorded in 37.5% of cases. Independent prognostic factors of RFS were the LNR (HR = 2.911; 95% CI = 1.468-5.779; p = 0.002), FIGO stage (HR = 1.835; 95% CI = 1.071-3.141; p = 0.027), and free margins (HR = 2.091; 95% CI = 1.286-3.999; p = 0.003).
Conclusion: Surgical margin, FIGO stage, and LNR represent the independent prognostic factors of survival and LNR showed superior prognostic predictive accuracy compared with the revised 2021 FIGO staging system for predicting OS and RFS in VSCC.
Keywords: lymph node metastases; lymph node ratio; prognosis; recurrence; vulvar cancer.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures





References
-
- Zapardiel I, Iacoponi S, Coronado PJ, et al. Prognostic factors in patients with vulvar cancer: the VULCAN study. Int J Gynecol Cancer 2020; 30(9): 1285–1291. - PubMed
-
- van der Steen S, de Nieuwenhof HP, Massuger L, et al. New FIGO staging system of vulvar cancer indeed provides a better reflection of prognosis. Gynecol Oncol 2010; 119(3): 520–125. - PubMed
-
- Baiocchi G, Silva Cestari FM, Rocha RM, et al. Prognostic value of the number and laterality of metastatic inguinal lymph nodes in vulvar cancer: revisiting the FIGO staging system. Eur J Surg Oncol 2013; 39(7): 780–785. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

