Clinical utility analysis of the Hoxb8 mast cell activation test for the diagnosis of peanut allergy
- PMID: 39340441
- PMCID: PMC11724244
- DOI: 10.1111/all.16341
Clinical utility analysis of the Hoxb8 mast cell activation test for the diagnosis of peanut allergy
Abstract
Background: Peanut allergy is among the most severe and common food allergies. The diagnosis has a significant impact on the quality of life for patients and their families. An effective management approach depends on accurate, safe, and easily implementable diagnostic methods. We previously developed a cell-based assay using Hoxb8 mast cells (Hoxb8 MCs) aimed at improving clinical allergy diagnosis. In this study, we assessed its diagnostic performance by measuring blinded sera from a prospectively enrolled and pre-validated peanut allergy cohort.
Methods: Hoxb8 MCs were passively sensitized with sera from peanut-allergic and peanut tolerant children and adolescents (n = 112). Degranulation of Hoxb8 MCs was quantified upon stimulation with dose-titrated peanut extract by means of flow cytometry, using CD107a as activation marker. The results from the Hoxb8 mast cell activation test (Hoxb8 MAT) were compared to established diagnostic assays such as the skin prick test (SPT), specific IgE (sIgE) levels, and the basophil activation test (BAT). Additionally, serum samples from BAT nonresponders were assessed with the Hoxb8 MAT.
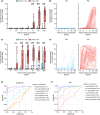
Results: Hoxb8 MAT displayed a robust dose-dependent activation to peanut extract, with a cutoff value of ≤5.2% CD107a positive cells. The diagnostic accuracy was highest at allergen concentrations ≥100 ng/mL, with an area under the receiver operating characteristic curve (AUROC) of 0.97, 93% sensitivity, and 96% specificity, outperforming traditional SPT and sIgE tests. When compared to BAT, Hoxb8 MAT exhibited comparable diagnostic efficacy. Moreover, sera from BAT nonresponders were accurately classified into allergics and nonallergics by the Hoxb8 MAT.
Conclusions: The Hoxb8 MAT demonstrated a very good diagnostic precision in patients prospectively assessed for peanut allergy comparable to the fresh whole blood-based BAT. Additionally, it demonstrated its value for accurate classification of BAT nonresponders into allergic and nonallergic individuals. Further investigations into its utility in the routine clinical setting are warranted.
Keywords: Hoxb8 mast cell activation test; basophil activation test; oral food challenge; peanut allergy diagnosis; specific IgE.
© 2024 The Author(s). Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
NBZ is a cofounder, shareholder, and employee of ATANIS Biotech AG. AC has nothing to declare. RvB is an employee of ATANIS Biotech AG. NR, MS, JAH, XY, CMD, and LD have nothing to disclose. JEMU reports grants and personal fees from ALK‐Abelló A/S, personal fees from Bausch Health, personal fees from Kaleo and Pharming Group N.V, grants from DBV Technologies, grants from Regeneron and Sanofi, and grants from Food Allergy Anaphylaxis Programme (SickKids), outside the submitted work; and Section Chair of Food Allergy and Anaphylaxis, Canadian Society of Allergy and Clinical Immunology; Healthcare Advisory Board, Food Allergy Canada. TK is a cofounder, shareholder, and board member of ATANIS Biotech AG. AE is a cofounder, consultant, and shareholder of ATANIS Biotech AG and Excellergy, Inc. AE received grants from ATANIS Biotech AG, Nestlé, Novartis AG, and BÜHLMANN Laboratories AG. TE reports grants from CIHR, FWF, Food Allergy and Anaphylaxis Program Sickkids, ALK. He is site PI of company sponsored trials by DBV, Novartis and Stallergenes Greer, EFSA and FARE. Personal fees from Danone/Nutricia/Milupa, ThermoFisher, Aimmune, Stallergenes Greer, ALK, MADx and Nonfinancial support from Novartis and MADx, all outside the submitted work. He is an associate editor of Allergy.
Figures




References
-
- Hourihane JO, Kilburn SA, Dean P, Warner JO. Clinical characteristics of peanut allergy. Clin Exp Allergy. 1997;27:634‐639. - PubMed
-
- Cummings AJ, Knibb RC, King RM, Lucas JS. The psychosocial impact of food allergy and food hypersensitivity in children, adolescents and their families: a review. Allergy. 2010;65:933‐945. - PubMed

