Epicutaneous immunotherapy for the treatment of peanut allergy
- PMID: 39340442
- PMCID: PMC11724258
- DOI: 10.1111/all.16324
Epicutaneous immunotherapy for the treatment of peanut allergy
Abstract
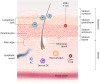
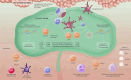
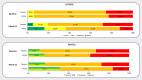
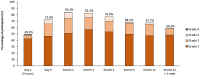
Peanut allergy treatment options remain limited, but novel approaches are being studied, including epicutaneous immunotherapy (EPIT). EPIT uses the cutaneous immune system to promote tolerance to food allergens. Viaskin™ Peanut, an approach to EPIT in late-stage clinical development uses an occlusive patch with a condensation chamber that enables natural epidermal water loss to solubilize dry antigen on the patch, which is then absorbed and captured by skin Langerhans cells. This form of EPIT does not require disruption of the skin barrier, thus avoiding a proinflammatory cytokine response by targeting the nonvascularized epidermis and limiting systemic allergen exposure. Extensive preclinical research suggests that Viaskin Peanut has a distinct mechanism of desensitization, including the potential for disease modification, driven by a unique population of regulatory T cells. Numerous clinical studies of Viaskin Peanut have demonstrated desensitization and reductions in reaction severity, particularly in children aged 1 through 11 years, as well as a favorable safety profile with mostly mild-to-moderate skin reactions that were observed to decrease over time. EPIT with Viaskin Peanut may be a potential therapeutic option for peanut allergy that is clinically practical with long-term efficacy and tolerability.
Keywords: Viaskin Peanut; epicutaneous immunotherapy; food allergy; peanut allergy.
© 2024 The Author(s). Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
M.R. has no disclosures to report. H.A.S. reports consulting fees from N‐Fold, LLC, DBV Technologies, AbbVie, Food Allergy Research and Education (FARE), and Siolta Therapeutics, stock options from N‐Fold, LLC, and DBV Technologies, grants to his institution from the National Institute of Allergy and Infectious Diseases, and FARE, royalties from Elsevier and Wiley, participated in a scientific advisory board for FARE and Siolta Therapeutics, received honoraria for American Academy of Allergy, Asthma, and Immunology, American College of Allergy, Asthma, and Immunology, and Greater Washington Allergy, Asthma and Immunology Society, received support to attend meetings from DBV Technologies, and has patents held by Mount Sinai. E.H.K. reports consulting fees from ALK‐Abelló, Ukko Inc., Cellergy Pharma, DBV Technologies, Genentech, Hanimune Therapeutics, Novartis, Nutricia, Phyalxis BioScience, and Revolo Biotherapeutics; safety review committee membership for Allergy Therapeutics Ltd.; royalties from UpToDate; and has stock options for Cellergy Pharma. K.J.B. and T.D.G. are employees of DBV Technologies. A.W.B. reports consulting fees from Allergy Therapeutics, Aimmune Therapeutics, Consortia TX, DBV Technologies, Ukko, grants to his institution from Burroughs Wellcome Fund, research grant from the National Institutes of Health, and royalties from UpToDate.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

