Ad26.COV2.S COVID-19 Vaccine Safety And Immunogenicity in Adolescents 16-17 Years of Age
- PMID: 39340467
- PMCID: PMC11599148
- DOI: 10.1093/jpids/piae098
Ad26.COV2.S COVID-19 Vaccine Safety And Immunogenicity in Adolescents 16-17 Years of Age
Abstract
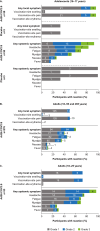
2.5 × 1010 vp Ad26.COV2.S elicited robust SARS-CoV-2-specific antibody responses in adolescents through 6 months, with acceptable safety and reactogenicity profiles. Compared with adults immunized with 5 × 1010 vp Ad26.COV2.S, adolescents had higher antibody levels, despite being vaccinated with a lower dose.
Keywords: Ad26.COV2.S; COVID-19; adolescents; infectious diseases; vaccine/immunization.
© The Author(s) 2024. Published by Oxford University Press on behalf of The Journal of the Pediatric Infectious Diseases Society.
Figures
References
-
- Hardt K, Vandebosch A, Sadoff J, et al. ; ENSEMBLE2 study group. Efficacy, safety, and immunogenicity of a booster regimen of Ad26.COV2.S vaccine against COVID-19 (ENSEMBLE2): results of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Infect Dis 2022; 22:1703–15. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

