TRPC3/6 Channels Mediate Mechanical Pain Hypersensitivity via Enhancement of Nociceptor Excitability and of Spinal Synaptic Transmission
- PMID: 39340833
- PMCID: PMC11600220
- DOI: 10.1002/advs.202404342
TRPC3/6 Channels Mediate Mechanical Pain Hypersensitivity via Enhancement of Nociceptor Excitability and of Spinal Synaptic Transmission
Abstract
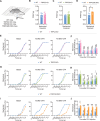
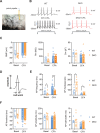
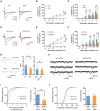
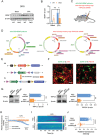
Patients with tissue inflammation or injury often experience aberrant mechanical pain hypersensitivity, one of leading symptoms in clinic. Despite this, the molecular mechanisms underlying mechanical distortion are poorly understood. Canonical transient receptor potential (TRPC) channels confer sensitivity to mechanical stimulation. TRPC3 and TRPC6 proteins, coassembling as heterotetrameric channels, are highly expressed in sensory neurons. However, how these channels mediate mechanical pain hypersensitivity has remained elusive. It is shown that in mice and human, TRPC3 and TRPC6 are upregulated in DRG and spinal dorsal horn under pathological states. Double knockout of TRPC3/6 blunts mechanical pain hypersensitivity, largely by decreasing nociceptor hyperexcitability and spinal synaptic potentiation via presynaptic mechanism. In corroboration with this, nociceptor-specific ablation of TRPC3/6 produces comparable pain relief. Mechanistic analysis reveals that upon peripheral inflammation, TRPC3/6 in primary sensory neurons get recruited via released bradykinin acting on B1/B2 receptors, facilitating BDNF secretion from spinal nociceptor terminals, which in turn potentiates synaptic transmission through TRPC3/6 and eventually results in mechanical pain hypersensitivity. Antagonizing TRPC3/6 in DRG relieves mechanical pain hypersensitivity in mice and nociceptor hyperexcitability in human. Thus, TRPC3/6 in nociceptors is crucially involved in pain plasticity and constitutes a promising therapeutic target against mechanical pain hypersensitivity with minor side effects.
Keywords: TRPC3; TRPC6; mechanical pain hypersensitivity; nociceptor; synaptic potentiation.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
- 2021ZD0203100(2021ZD0203104)/STI2030-Major Projects
- 2021ZD0203200(2021ZD0203205)/STI2030-Major Projects
- 82330036/Natural Science Foundation of China
- 32071002/Natural Science Foundation of China
- 82171212/Natural Science Foundation of China
- 82371225/Natural Science Foundation of China
- 82221001/Natural Science Foundation of China
- 82101293/Natural Science Foundation of China
- 82201368/Natural Science Foundation of China
- 82201370/Natural Science Foundation of China
- 2022TD-49/Innovation Team Accredited by Shaanxi Science and Technology
- Z01-ES-101684/Intramural Research Program of the NIH
- (LHJJ24JH08)/Joint Founding Project of Innovation Research Institute, Xijing Hospital
LinkOut - more resources
Full Text Sources
