Ex vivo modelling of cardiac injury identifies ferroptosis-related pathways as a potential therapeutic avenue for translational medicine
- PMID: 39341589
- PMCID: PMC7617241
- DOI: 10.1016/j.yjmcc.2024.09.012
Ex vivo modelling of cardiac injury identifies ferroptosis-related pathways as a potential therapeutic avenue for translational medicine
Abstract
Background: Heart failure (HF) is a burgeoning health problem worldwide. Often arising as a result of cardiac injury, HF has become a major cause of mortality with limited availability of effective treatments. Ferroptotic pathways, triggering an iron-dependent form of cell death, are known to be potential key players in heart disease. This form of cell death does not exhibit typical characteristics of programmed cell death, and is mediated by impaired iron metabolism and lipid peroxidation signalling.
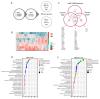
Objectives: The aim of this study is to establish an ex-vivo model of myocardial injury in living myocardial slices (LMS) and to identify novel underlying mechanisms and potential therapeutic druggable target(s).
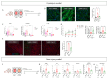
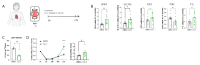
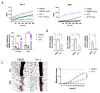
Methods and results: In this study, we employed LMS as an ex vivo model of cardiac injury to investigate underlying mechanisms and potential therapeutic targets. Cryoinjury was induced in adult rat LMS, resulting in 30 % tissue damage. Cryoinjured LMS demonstrated impaired contractile function, cardiomyocyte hypertrophy, inflammation, and cardiac fibrosis, closely resembling in vivo cardiac injury characteristics. Proteomic analysis revealed an enrichment of factors associated with ferroptosis in the injured LMS, suggesting a potential causative role. To test this hypothesis, we pharmacologically inhibited ferroptotic pathways using ferrostatin (Fer-1) in the cryoinjured rat LMS, resulting in attenuation of structural changes and repression of pro-fibrotic processes. Furthermore, LMS generated from failing human hearts were used as a model of chronic heart failure. In this model, Fer-1 treatment was observed to reduce the expression of ferroptotic genes, enhances contractile function and improves tissue viability. Blocking ferroptosis-associated pathways in human cardiac fibroblasts (HCFs) resulted in a downregulation of fibroblast activation genes, a decrease in fibroblast migration capacity, and a reduction in reactive oxygen species production. RNA sequencing analysis of Fer-1-treated human LMS implicated metallothioneins as a potential underlying mechanism for the inhibition of these pathways. This effect is possibly mediated through the replenishment of glutathione reserves.
Conclusions: Our findings highlight the potential of targeting ferroptosis-related pathways and metallothioneins as a promising strategy for the treatment of heart disease.
Keywords: Cardiac fibroblast; Cardiac injury; Ferrostatin; Living myocardial slice; Metallothionein.
Copyright © 2024 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest T. Thum is founder and CSO/CMO of Cardior Pharmaceuticals GmbH, a wholly-owned subsidiary of Novo Nordisk Europe A/S (outside of this study). The other authors declare that they have no conflict of interest.
Figures


References
-
- Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, et al. Heart disease and stroke Statistics-2023 update: a report from the American Heart Association. Circulation. 2023 - PubMed
-
- Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000;102:470–479. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

