Improving the alcohol respiratory chain and energy metabolism by enhancing PQQ synthesis in Acetobacter pasteurianus
- PMID: 39341788
- PMCID: PMC11503474
- DOI: 10.1093/jimb/kuae036
Improving the alcohol respiratory chain and energy metabolism by enhancing PQQ synthesis in Acetobacter pasteurianus
Abstract
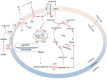
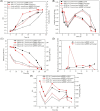
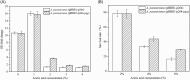
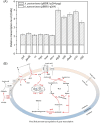
Pyrroloquinoline quinone (PQQ) is one of the important coenzymes in living organisms. In acetic acid bacteria (AAB), it plays a crucial role in the alcohol respiratory chain, as a coenzyme of alcohol dehydrogenase (ADH). In this work, the PQQ biosynthetic genes were overexpressed in Acetobacter pasteurianus CGMCC 3089 to improve the fermentation performance. The result shows that the intracellular and extracellular PQQ contents in the recombinant strain A. pasteurianus (pBBR1-p264-pqq) were 152.53% and 141.08% higher than those of the control A. pasteurianus (pBBR1-p264), respectively. The catalytic activity of ADH and aldehyde dehydrogenase increased by 52.92% and 67.04%, respectively. The results indicated that the energy charge and intracellular ATP were also improved in the recombinant strain. The acetic acid fermentation was carried out using a 5 L self-aspirating fermenter, and the acetic acid production rate of the recombinant strain was 23.20% higher compared with the control. Furthermore, the relationship between the PQQ and acetic acid tolerance of cells was analyzed. The biomass of recombinant strain was 180.2%, 44.3%, and 38.6% higher than those of control under 2%, 3%, and 4% acetic acid stress, respectively. After being treated with 6% acetic acid for 40 min, the survival rate of the recombinant strain was increased by 76.20% compared with the control. Those results demonstrated that overexpression of PQQ biosynthetic genes increased the content of PQQ, therefore improving the acetic acid fermentation and the cell tolerance against acetic acid by improving the alcohol respiratory chain and energy metabolism.
One sentence summary: The increase in PQQ content enhances the activity of the alcohol respiratory chain of Acetobacter pasteurianus, and the increase in energy charge enhances the tolerance of cells against acetic acid, therefore, improving the efficiency of acetic acid fermentation.
Keywords: Acetobacter pasteurianus; Acetic acid fermentation; Acetic acid tolerance; Energy charge; pyrroloquinoline quinone.
© The Author(s) 2024. Published by Oxford University Press on behalf of Society of Industrial Microbiology and Biotechnology.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Acetobacter pasteurianus metabolic change induced by initial acetic acid to adapt to acetic acid fermentation conditions.Appl Microbiol Biotechnol. 2017 Sep;101(18):7007-7016. doi: 10.1007/s00253-017-8453-8. Epub 2017 Aug 2. Appl Microbiol Biotechnol. 2017. PMID: 28770302
-
Producing Acetic Acid of Acetobacter pasteurianus by Fermentation Characteristics and Metabolic Flux Analysis.Appl Biochem Biotechnol. 2018 Sep;186(1):217-232. doi: 10.1007/s12010-018-2732-4. Epub 2018 Mar 19. Appl Biochem Biotechnol. 2018. PMID: 29552715
-
Major aldehyde dehydrogenase AldFGH of Gluconacetobacter diazotrophicus is independent of pyrroloquinoline quinone but dependent on molybdopterin for acetic acid fermentation.Appl Microbiol Biotechnol. 2021 Mar;105(6):2341-2350. doi: 10.1007/s00253-021-11144-x. Epub 2021 Feb 16. Appl Microbiol Biotechnol. 2021. PMID: 33591385
-
Impacts of bioprocess engineering on product formation by Acetobacter pasteurianus.Appl Microbiol Biotechnol. 2018 Mar;102(6):2535-2541. doi: 10.1007/s00253-018-8819-6. Epub 2018 Feb 11. Appl Microbiol Biotechnol. 2018. PMID: 29430583 Review.
-
Pyrroloquinoline quinone-dependent dehydrogenases of acetic acid bacteria.Appl Microbiol Biotechnol. 2018 Nov;102(22):9531-9540. doi: 10.1007/s00253-018-9360-3. Epub 2018 Sep 15. Appl Microbiol Biotechnol. 2018. PMID: 30218379 Review.
References
-
- Azuma Y., Hosoyama A., Matsutani M., Furuya N., Horikawa H., Harada T., Hirakawa H., Kuhara S., Matsushita K., Fujita N., Shirai M. (2009). Whole-genome analyses reveal genetic instability of Acetobacter pasteurianus. Nucleic Acids Research, 37(17), 5768–5783. 10.1093/nar/gkp612 - DOI - PMC - PubMed
-
- Chinnawirotpisan P., Theeragool G., Limtong S., Toyama H., Adachi O. O., Matsushita K. (2003). Quinoprotein alcohol dehydrogenase is involved in catabolic acetate production, while NAD-dependent alcohol dehydrogenase in ethanol assimilation in Acetobacter pasteurianus SKU1108. Journal of Bioscience and Bioengineering, 96(6), 564–571. 10.1016/S1389-1723(04)70150-4 - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

