Cost-effectiveness of a novel AI technology to quantify coronary inflammation and cardiovascular risk in patients undergoing routine coronary computed tomography angiography
- PMID: 39341792
- PMCID: PMC12187117
- DOI: 10.1093/ehjqcco/qcae085
Cost-effectiveness of a novel AI technology to quantify coronary inflammation and cardiovascular risk in patients undergoing routine coronary computed tomography angiography
Abstract
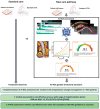
Aims: Coronary computed tomography angiography (CCTA) is a first-line investigation for chest pain in patients with suspected obstructive coronary artery disease (CAD). However, many acute cardiac events occur in the absence of obstructive CAD. We assessed the lifetime cost-effectiveness of integrating a novel artificial intelligence-enhanced image analysis algorithm (AI-Risk) that stratifies the risk of cardiac events by quantifying coronary inflammation, combined with the extent of coronary artery plaque and clinical risk factors, by analysing images from routine CCTA.
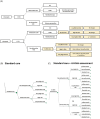
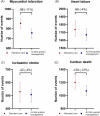
Methods and results: A hybrid decision-tree with population cohort Markov model was developed from 3393 consecutive patients who underwent routine CCTA for suspected obstructive CAD and followed up for major adverse cardiac events over a median (interquartile range) of 7.7(6.4-9.1) years. In a prospective real-world evaluation survey of 744 consecutive patients undergoing CCTA for chest pain investigation, the availability of AI-Risk assessment led to treatment initiation or intensification in 45% of patients. In a further prospective study of 1214 consecutive patients with extensive guidelines recommended cardiovascular risk profiling, AI-Risk stratification led to treatment initiation or intensification in 39% of patients beyond the current clinical guideline recommendations. Treatment guided by AI-Risk modelled over a lifetime horizon could lead to fewer cardiac events (relative reductions of 11%, 4%, 4%, and 12% for myocardial infarction, ischaemic stroke, heart failure, and cardiac death, respectively). Implementing AI-Risk Classification in routine interpretation of CCTA is highly likely to be cost-effective (incremental cost-effectiveness ratio £1371-3244), both in scenarios of current guideline compliance, or when applied only to patients without obstructive CAD.
Conclusions: Compared with standard care, the addition of AI-Risk assessment in routine CCTA interpretation is cost-effective, by refining risk-guided medical management.
Keywords: Coronary CT angiography; Coronary artery disease; Cost-effectiveness analysis; Inflammation.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: S.F., N.S., P.T., Y.S., and M.S. are employees of Caristo Diagnostics Ltd. S.N., K.M.C., and C.A. are founders, shareholders, and directors of Caristo Diagnostics Ltd, a CT-image analysis company. C.A. is the inventor of patents US10695023B2, US11393137B2, GB2018/1818049.7, GR20180100490, and GR20180100510. S.N. and K.M.C. are co-inventors of patent US10695023B2. These are licensed to Caristo Diagnostics by the University of Oxford. A.T. has received research funding from NIHR Oxford Health Biomedical Research Center, NHS AI award, and NIHR Applied Research Collaboration Oxford. A.K. has received grants from Lantheus Medical USA, and honoraria from Bracco UK Ltd/Philips Medical Ltd. B.M. has received honoraria from Chiesi, Sanofi, Novartis, and Boston Scientific. C.A. has a leadership role in British Atherosclerosis Society, EU Marie Curie Fellowship committee, and received honoraria from Amarin, Covance, and consulting fees from Slience Therapeutics. D.A. has a leadership role in Spontaneous Coronary Artery Dissection Study group, inventor of patents related to cardiac assist device (EP3277337A1, PCT/GB2017/050877) and received grant support from AstraZeneca, Abbott Vascular, and consulting fees from General Electric Inc. E.M. has received research support from NHS AI award. E.N. has a leadership role in Society of Cardiac CT and has received consulting fees from Caristo Diagnostics. J.D. has a leadership role and received consulting fees from Novo Nordisk, and received honoraria from Amgen, Boehringer Ingelheim, Merck, Pfizer, Aegerion, Novartis, Sanofi, Takeda, Novo Nordisk, and Bayer. J.R. has a leadership role in Heart and Lung Imaging LTD, and received consulting fees from NHSX and HeartFlow, and honoraria from Sanofi, Aidence, 4-C. K.M.C. has received consulting fees from Caristo Diagnostics, M.D. has received consulting fees from Bristol Myers Squibb, Tenaya Therapeutics, and VizAL, and participated on advisory board for Caristo Diagnostics. N.S. has received royalties from patent (PCT/GB2015/052359). R.A. is an employee of Lumanity, a consultancy company that has been contracted to provide advice on the conduct and reporting of this study. R.B. has a leadership role in Society of Cardiovascular Computed Tomography, has received grants from Amgen, Novartis, and Nanox AI, and consulting fees from Caristo Diagnostics and Heartflow. All other authors declare no competing interests.
Figures


References
-
- Luengo-Fernandez R, Walli-Attaei M, Gray A, Torbica A, Maggioni AP, Huculeci R et al. Economic burden of cardiovascular diseases in the European Union: a population-based cost study. Eur Heart J 2021, 144, 2014, 7, 276–284, 2003, 114, 485–494, 2007, 5, 5, 2023;44:4752–4767. 10.1093/eurheartj/ehad583 - DOI - PMC - PubMed
-
- NICE . Cardiovascular Disease: Risk Assessment and Reduction, Including Lipid Modification NICE Guideline [NG238]. Available from: https://www.nice.org.uk/guidance/ng238 (accessed 17 October 2024).
MeSH terms
Grants and funding
- AI_AWARD02443/NHS-AI
- AI_AWARD02013/NHS-AI
- CH/F/21/90009/BHF_/British Heart Foundation/United Kingdom
- CH/16/1/32013/BHF_/British Heart Foundation/United Kingdom
- TG/19/2/34831/BHF_/British Heart Foundation/United Kingdom
- RG/F/21/110040/BHF_/British Heart Foundation/United Kingdom
- 104472/Innovate UK
- 104688/WT_/Wellcome Trust/United Kingdom
- National Consortium of Intelligent Medical Imaging
- 965286/EU Research and Innovation Action MAESTRIA
- NIHR Oxford Biomedical Research Centre
- RG/18/3/34214/British Heart Foundation Oxford Centre of Research Excellence

