The urotensin II receptor triggers an early meningeal response and a delayed macrophage-dependent vasospasm after subarachnoid hemorrhage in male mice
- PMID: 39341842
- PMCID: PMC11439053
- DOI: 10.1038/s41467-024-52654-2
The urotensin II receptor triggers an early meningeal response and a delayed macrophage-dependent vasospasm after subarachnoid hemorrhage in male mice
Abstract
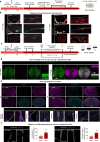
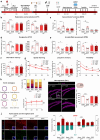
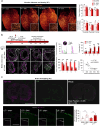
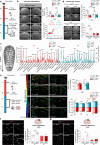
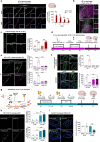
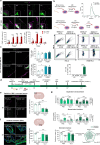
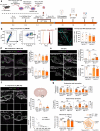
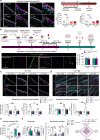
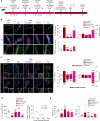
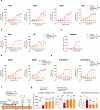
Subarachnoid hemorrhage (SAH) can be associated with neurological deficits and has profound consequences for mortality and morbidity. Cerebral vasospasm (CVS) and delayed cerebral ischemia affect neurological outcomes in SAH patients, but their mechanisms are not fully understood, and effective treatments are limited. Here, we report that urotensin II receptor UT plays a pivotal role in both early events and delayed mechanisms following SAH in male mice. Few days post-SAH, UT expression is triggered by blood or hemoglobin in the leptomeningeal compartment. UT contributes to perimeningeal glia limitans astrocyte reactivity, microvascular alterations and neuroinflammation independent of CNS-associated macrophages (CAMs). Later, CAM-dependent vascular inflammation and subsequent CVS develop, leading to cognitive dysfunction. In an SAH model using humanized UTh+/h+ male mice, we show that post-SAH CVS and behavioral deficits, mediated by UT through Gq/PLC/Ca2+ signaling, are prevented by UT antagonists. These results highlight the potential of targeting UT pathways to reduce early meningeal response and delayed cerebral ischemia in SAH patients.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

