Effectiveness of data-augmentation on deep learning in evaluating rapid on-site cytopathology at endoscopic ultrasound-guided fine needle aspiration
- PMID: 39341885
- PMCID: PMC11439075
- DOI: 10.1038/s41598-024-72312-3
Effectiveness of data-augmentation on deep learning in evaluating rapid on-site cytopathology at endoscopic ultrasound-guided fine needle aspiration
Abstract
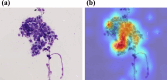
Rapid on-site cytopathology evaluation (ROSE) has been considered an effective method to increase the diagnostic ability of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA); however, ROSE is unavailable in most institutes worldwide due to the shortage of cytopathologists. To overcome this situation, we created an artificial intelligence (AI)-based system (the ROSE-AI system), which was trained with the augmented data to evaluate the slide images acquired by EUS-FNA. This study aimed to clarify the effects of such data-augmentation on establishing an effective ROSE-AI system by comparing the efficacy of various data-augmentation techniques. The ROSE-AI system was trained with increased data obtained by the various data-augmentation techniques, including geometric transformation, color space transformation, and kernel filtering. By performing five-fold cross-validation, we compared the efficacy of each data-augmentation technique on the increasing diagnostic abilities of the ROSE-AI system. We collected 4059 divided EUS-FNA slide images from 36 patients with pancreatic cancer and nine patients with non-pancreatic cancer. The diagnostic ability of the ROSE-AI system without data augmentation had a sensitivity, specificity, and accuracy of 87.5%, 79.7%, and 83.7%, respectively. While, some data-augmentation techniques decreased diagnostic ability, the ROSE-AI system trained only with the augmented data using the geometric transformation technique had the highest diagnostic accuracy (88.2%). We successfully developed a prototype ROSE-AI system with high diagnostic ability. Each data-augmentation technique may have various compatibilities with AI-mediated diagnostics, and the geometric transformation was the most effective for the ROSE-AI system.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Yoshinaga, S. et al. Safety and efficacy of endoscopic ultrasound-guided fine needle aspiration for pancreatic masses: a prospective multicenter study. Dig. Endosc.32, 114–126 (2020). - PubMed
-
- Dumonceau, J. M. et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline-updated January 2017. Endoscopy49(07), 695–714 (2017). - PubMed
-
- Bang, J. Y., Hawes, R. & Varadarajulu, S. A meta-analysis comparing Procore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy48, 339–349 (2016). - PubMed
-
- Hawes, R. H. The evolution of endoscopic ultrasound: improved imaging, higher accuracy for fine needle aspiration and the reality of endoscopic ultrasound-guided interventions. Curr. Opin. Gastroenterol.26, 436–444 (2010). - PubMed
-
- Schmidt, R. L., Walker, B. S., Howard, K., Layfield, L. J. & Adler, D. G. Rapid on-site evaluation reduces needle passes in endoscopic ultrasound-guided fine-needle aspiration for solid pancreatic lesions: a risk benefit analysis. Dig. Dis. Sci.58, 3280–3286 (2013). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

