MiRNA expression profiling reveals a potential role of microRNA-148b-3p in cerebral vasospasm in subarachnoid hemorrhage
- PMID: 39341923
- PMCID: PMC11438990
- DOI: 10.1038/s41598-024-73579-2
MiRNA expression profiling reveals a potential role of microRNA-148b-3p in cerebral vasospasm in subarachnoid hemorrhage
Abstract
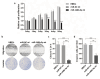
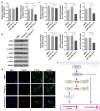
Cerebral vasospasm (CVS) is an important contributor to delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage (aSAH), leading to high morbidity and long-term disability. While several microRNAs (miRNAs) have been implicated in vasospasm, the underlying mechanisms for CVS remain poorly understood. Our study aims to identify miRNAs that may contribute to the development of CVS. Whole-blood samples were obtained during or outside of vasospasm from aSAH patients whose maximal vasospasm was moderate or severe. MiRNAs were isolated from serial whole-blood samples, and miRNA sequencing was performed. Differentially expressed miRNAs were identified and the expression levels in patients' samples were verified using real-time qPCR. The biological functions of identified miRNA were evaluated in human brain endothelial cells (HBECs). MiRNA profiling revealed significant upregulation of miR-148b-3p in patients during CVS. We demonstrated that miR-148b-3p directly targeted and decreased the expression of ROCK1, affecting cell proliferation, migration, and invasion of HBECs through the ROCK-LIMK-Cofilin pathway. We propose that the upregulation of miRNA-148b-3p plays a role in the development of CVS by regulating actin cytoskeletal dynamics in HBECs, which is crucial for vascular function. Our study highlights miR-148b-3p as a potential diagnostic marker as well as therapeutic target for CVS following aSAH.
Keywords: Aneurysmal subarachnoid hemorrhage; Cerebral vasospasm; MiRNA profiling; MiRNA-148-3p; ROCK-LIMK-Cofilin pathway.
© 2024. The Author(s).
Conflict of interest statement
MAA: Proctor for Covidien and Codman. The other authors declare no competing interests.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

