A multi-epitope protein vaccine encapsulated in alginate nanoparticles as a candidate vaccine against Shigella sonnei
- PMID: 39341926
- PMCID: PMC11438873
- DOI: 10.1038/s41598-024-73105-4
A multi-epitope protein vaccine encapsulated in alginate nanoparticles as a candidate vaccine against Shigella sonnei
Abstract
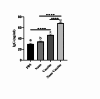
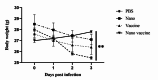
Shigellosis, caused by the Gram-negative bacterium Shigella, is a major global health challenge. Despite extensive research over the past two decades, no commercial vaccine is available to prevent Shigella infection. Developing multi-epitope vaccines offers a promising and innovative approach to tackling infectious diseases. In this study, we produced a multi-epitope vaccine candidate using E. coli BL21 (DE3) plysS bacteria and purified the vaccine protein with Ni-NTA affinity chromatography. We then prepared alginate nanoparticles containing the vaccine protein, with a particle size of 122 ± 6 nm, PDI 0.17, SPAN 0.83, and zeta potential of -27 ± 2 mV. Successful protein loading was confirmed through nanodrop and ATR-FTIR analyses. To evaluate the immunogenicity of the encapsulated vaccine, mice were orally vaccinated, and their serum was analyzed for IgG, IL-4, and IFN-γ levels cytokines. The results showed a significant increase in IgG level in the vaccinated group compared to controls. Additionally, the vaccinated group exhibited a notable increase in IL-4 and IFN-γ cytokines, indicating a robust Th-cell-mediated immune response essential for combating Shigella. Our nano-vaccine demonstrated high efficacy in activating both humoral and cellular immunity, effectively protecting against the bacteria. The alginate-based oral vaccine candidate thus emerges as a promising strategy for developing a multi-epitope vaccine candidate against Shigella.
Keywords: Alginate nanoparticles; Multi-epitope; Oral vaccination; Shigellosis; Vaccine.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Muthuirulandi Sethuvel, D., Devanga Ragupathi, N., Anandan, S. & Veeraraghavan, B. Update on: Shigella new serogroups/serotypes and their antimicrobial resistance. Lett. Appl. Microbiol.64 (1), 8–18 (2017). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

