Development and validation of RT-LAMP for detecting yellow fever virus in non-human primates samples from Brazil
- PMID: 39342022
- PMCID: PMC11438901
- DOI: 10.1038/s41598-024-74020-4
Development and validation of RT-LAMP for detecting yellow fever virus in non-human primates samples from Brazil
Abstract
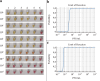
Monitoring yellow fever in non-human primates (NHPs) is an early warning system for sylvatic yellow fever outbreaks, aiding in preventing human cases. However, current diagnostic tests for this disease, primarily relying on RT-qPCR, are complex and costly. Therefore, there is a critical need for simpler and more cost-effective methods to detect yellow fever virus (YFV) infection in NHPs, enabling early identification of viral circulation. In this study, an RT-LAMP assay for detecting YFV in NHP samples was developed and validated. Two sets of RT-LAMP primers targeting the YFV NS5 and E genes were designed and tested together with a third primer set to the NS1 locus using NHP tissue samples from Southern Brazil. The results were visualized by colorimetry and compared to the RT-qPCR test. Standardization and validation of the RT-LAMP assay demonstrated 100% sensitivity and specificity compared to RT-qPCR, with a detection limit of 12 PFU/mL. Additionally, the cross-reactivity test with other flaviviruses confirmed a specificity of 100%. Our newly developed RT-LAMP diagnostic test for YFV in NHP samples will significantly contribute to yellow fever monitoring efforts, providing a simpler and more accessible method for viral early detection. This advancement holds promise for enhancing surveillance and ultimately preventing the spread of yellow fever.
Keywords: Diagnosis; RT-LAMP; Yellow fever; Yellow fever virus.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Vasconcelos, P. F. C. Febre amarela [yellow fever]. Rev. Soc. Bras. Med. Trop.36, 275–293 (2003). - PubMed
-
- Mukhopadhyay, S., Kuhn, R. J. & Rossmann, M. G. A. Structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol.3, 13–22 (2005). - PubMed
-
- Domingo, C., Patel, P., Linke, S., Achazi, K. & Niedrig, M. Molecular diagnosis of flaviviruses. Future Virol.6, 1059–1074 (2011).
-
- Chambers, T. J., Hahn, C. S., Galler, R. & Rice, C. M. Flavivirus genome organization, expression and replication. Annu. Rev. Microbiol.44, 649–688 (1990). - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

