Single-cell transcriptomics reveals e-cigarette vapor-induced airway epithelial remodeling and injury
- PMID: 39342154
- PMCID: PMC11439300
- DOI: 10.1186/s12931-024-02962-4
Single-cell transcriptomics reveals e-cigarette vapor-induced airway epithelial remodeling and injury
Abstract
Background: In recent years, e-cigarettes have been used as alternatives among adult smokers. However, the impact of e-cigarette use on human bronchial epithelial (HBE) cells remains controversial.
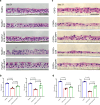
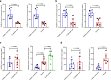
Methods: We collected primary HBE cells of healthy nonsmokers and chronic obstructive pulmonary disease (COPD) smokers, and analyzed the impact of e- cigarette vapor extract (ECE) or cigarette smoke extract (CSE) on HBE cell differentiation and injury by single-cell RNA sequencing, immunostaining, HE staining, qPCR and ELISA. We obtained serum and sputum from healthy non- smokers, smokers and e-cigarette users, and analyzed cell injury markers and mucin proteins.
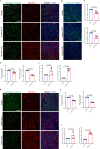
Results: ECE treatment led to a distinct differentiation program of ciliated cells and unique patterns of their cell-cell communications compared with CSE. ECE treatment caused increased Notch signaling strength in a ciliated cell subpopulation, and HBE cell remodeling and injury including hypoplasia of ciliated cells and club cells, and shorter cilia. ECE-induced hypoplasia of ciliated cells and shorter cilia were ameliorated by the Notch signaling inhibition.
Conclusions: This study reveals distinct characteristics in e-cigarette vapor-induced airway epithelial remodeling, pointing to Notch signaling pathway as a potential targeted intervention for e-cigarette vapor-caused ciliated cell differentiation defects and cilia injury. In addition, a decrease in SCGB1A1 proteins is associated with e- cigarette users, indicating a potential lung injury marker for e-cigarette users.
Keywords: COPD; Cilia; Ciliated cells; E-cigarette; Notch signaling.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests. At the time of the research, Zehong Wu and Xingtao Jiang were employees of RELX Science Center, Shenzhen RELX Tech.
Figures





Similar articles
-
Comparative effects of e-cigarette and conventional cigarette smoke on in vitro bronchial epithelial cell responses.Toxicol Lett. 2025 May 1;407:32-40. doi: 10.1016/j.toxlet.2025.03.003. Epub 2025 Mar 16. Toxicol Lett. 2025. PMID: 40101882
-
Cigarette Smoke Activates NOTCH3 to Promote Goblet Cell Differentiation in Human Airway Epithelial Cells.Am J Respir Cell Mol Biol. 2021 Apr;64(4):426-440. doi: 10.1165/rcmb.2020-0302OC. Am J Respir Cell Mol Biol. 2021. PMID: 33444514 Free PMC article.
-
Epithelial to mesenchymal transition is increased in patients with COPD and induced by cigarette smoke.Thorax. 2013 May;68(5):410-20. doi: 10.1136/thoraxjnl-2012-201761. Epub 2013 Jan 7. Thorax. 2013. PMID: 23299965
-
Bronchial Epithelial Calcium Metabolism Impairment in Smokers and Chronic Obstructive Pulmonary Disease. Decreased ORAI3 Signaling.Am J Respir Cell Mol Biol. 2019 Oct;61(4):501-511. doi: 10.1165/rcmb.2018-0228OC. Am J Respir Cell Mol Biol. 2019. PMID: 30943377
-
[Cell-molecular mechanisms of nitrogen dioxide-induced damaging effect on bronchial epithelium].Ross Fiziol Zh Im I M Sechenova. 2014 Aug;100(8):897-905. Ross Fiziol Zh Im I M Sechenova. 2014. PMID: 25682681 Review. Russian.
Cited by
-
An integrated machine learning model of transcriptomic genes in multi-center chronic obstructive pulmonary disease reveals the causal role of TIMP4 in airway epithelial cell.Respir Res. 2025 Apr 23;26(1):158. doi: 10.1186/s12931-025-03238-1. Respir Res. 2025. PMID: 40269868 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

