The reporting quality of meta-epidemiological studies needs substantial improvement: a research on research study
- PMID: 39342302
- PMCID: PMC11438193
- DOI: 10.1186/s13643-024-02661-7
The reporting quality of meta-epidemiological studies needs substantial improvement: a research on research study
Abstract
Background: Meta-epidemiological research plays a vital role in providing empirical evidence needed to develop methodological manuals and tools, but the reporting quality has not been comprehensively assessed, and the influence of reporting guidelines remains unclear. The current study aims to evaluate the reporting quality of meta-epidemiological studies, assess the impact of reporting guidelines, and identify factors influencing reporting quality.
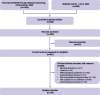
Methods: We searched PubMed and Embase for meta-epidemiological studies. The reporting quality of these studies was assessed for adherence to established reporting guidelines. Two researchers independently screened the studies and assessed the quality of the included studies. Time-series segmented linear regression was used to evaluate changes in reporting quality over time, while beta-regression analysis was performed to identify factors significantly associated with reporting quality.
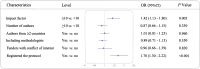
Results: We initially identified 1720 articles, of which 125 meta-epidemiological studies met the inclusion criteria. Of these, 65 (52%) had low reporting quality, 60 (48%) had moderate quality, and none achieved high quality. Of the 24 items derived from established reporting guidelines, 4 had poor adherence, 13 had moderate adherence, and 7 had high adherences. High journal impact factor (≥ 10) (OR = 1.42, 95% CI: 1.13, 1.80; P = 0.003) and protocol registration (OR = 1.70, 95% CI: 1.30, 2.22; P < 0.001) were significantly associated with better reporting quality. The publication of the reporting guideline did not significantly increase the mean reporting quality score (- 0.53, 95% CI: - 3.37, 2.31; P = 0.67) or the trend (- 0.38, 95% CI: - 1.02, 0.26; P = 0.20).
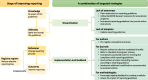
Conclusions: Our analysis showed suboptimal reporting quality in meta-epidemiological studies, with no improvement post-2017 guidelines. This potential shortcoming could hinder stakeholders' ability to draw reliable conclusions from these studies. While preregistration could reduce reporting bias, its adoption remains low. Registration platforms could consider creating tailored types for meta-epidemiological research, and journals need to adopt more proactive measures to enforce reporting standards.
Keywords: Interrupted time-series analysis; Meta-epidemiological studies; Reporting quality.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Sterne JAC, Jüni P, Schulz KF, Altman DG, Bartlett C, Egger M. Statistical methods for assessing the influence of study characteristics on treatment effects in ‘meta-epidemiological’ research. Stat Med. 2002;21(11):1513–24. - PubMed
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook For Systematic Reviews Of Interventions version 6.3 (updated February 2022). Cochrane. 2022;www.training.cochrane.org/handbook. Accessed 25 Jun 2024.
-
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366: l4898. - PubMed
-
- Saldanha IJ, Adam GP, Bañez LL, Bass EB, Berliner E, Devine B, et al. Inclusion of nonrandomized studies of interventions in systematic reviews of interventions: updated guidance from the Agency for Health Care Research and Quality Effective Health Care program. J Clin Epidemiol. 2022;152:300–6. - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

