SIRT6 modulates lesion microenvironment in LPC induced demyelination by targeting astrocytic CHI3L1
- PMID: 39342313
- PMCID: PMC11438192
- DOI: 10.1186/s12974-024-03241-1
SIRT6 modulates lesion microenvironment in LPC induced demyelination by targeting astrocytic CHI3L1
Abstract
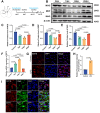
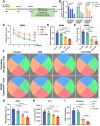
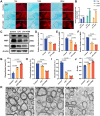
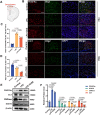
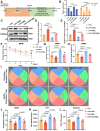
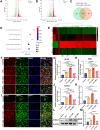
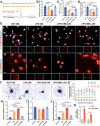
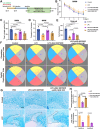
Demyelination occurs widely in the central nervous system (CNS) neurodegenerative diseases, especially the multiple sclerosis (MS), which with a complex and inflammatory lesion microenvironment inhibiting remyelination. Sirtuin6 (SIRT6), a histone/protein deacetylase is of interest for its promising effect in transcriptional regulation, cell cycling, inflammation, metabolism and longevity. Here we show that SIRT6 participates in the remyelination process in mice subjected to LPC-induced demyelination. Using pharmacological SIRT6 inhibitor or activator, we found that SIRT6 modulated LPC-induced damage in motor or cognitive function. Inhibition of SIRT6 impaired myelin regeneration, exacerbated neurological deficits, and decreased oligodendrocyte precursor cells (OPCs) proliferation and differentiation, whereas activation of SIRT6 reversed behavioral performance in mice, demonstrating a beneficial effect of SIRT6. Importantly, based on RNA sequencing analysis of the corpus callosum tissues, it was further revealed that SIRT6 took charge in regulation of glial activation during remyelination, and significant alterations in CHI3L1 were obtained, a glycoprotein specifically secreted by astrocytes. Impaired proliferation and differentiation of OPCs could be induced in vitro using supernatants from reactive astrocyte, especially when SIRT6 was inhibited. Mechanistically, SIRT6 regulates the secretion of CHI3L1 from reactive astrocytes by histone-H3-lysine-9 acetylation (H3K9Ac). Adeno-associated virus-overexpression of SIRT6 (AAV-SIRT6-OE) in astrocytes improved remyelination and functional recovery after LPC-induced demyelination, whereas together with AAV-CHI3L1-OE inhibits this therapeutic effect. Collectively, our data elucidate the role of SIRT6 in remyelination and further reveal astrocytic SIRT6/CHI3L1 as the key regulator for improving the remyelination environment, which may be a potential target for MS therapy.
Keywords: Astrocyte; CHI3L1; Multiple sclerosis; Remyelination; SIRT6.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Deletion of Voltage-Gated Calcium Channels in Astrocytes during Demyelination Reduces Brain Inflammation and Promotes Myelin Regeneration in Mice.J Neurosci. 2020 Apr 22;40(17):3332-3347. doi: 10.1523/JNEUROSCI.1644-19.2020. Epub 2020 Mar 13. J Neurosci. 2020. PMID: 32169969 Free PMC article.
-
Detrimental and protective action of microglial extracellular vesicles on myelin lesions: astrocyte involvement in remyelination failure.Acta Neuropathol. 2019 Dec;138(6):987-1012. doi: 10.1007/s00401-019-02049-1. Epub 2019 Jul 30. Acta Neuropathol. 2019. PMID: 31363836 Free PMC article.
-
Vagus nerve stimulation enhances remyelination and decreases innate neuroinflammation in lysolecithin-induced demyelination.Brain Stimul. 2024 May-Jun;17(3):575-587. doi: 10.1016/j.brs.2024.04.012. Epub 2024 Apr 20. Brain Stimul. 2024. PMID: 38648972
-
The Role of Astrocytes in Remyelination.Trends Neurosci. 2020 Aug;43(8):596-607. doi: 10.1016/j.tins.2020.05.006. Epub 2020 Jul 1. Trends Neurosci. 2020. PMID: 32620289 Review.
-
CHI3L1 in Multiple Sclerosis-From Bench to Clinic.Cells. 2024 Dec 17;13(24):2086. doi: 10.3390/cells13242086. Cells. 2024. PMID: 39768177 Free PMC article. Review.
References
-
- Woo MS, Engler JB, Friese MA. The neuropathobiology of multiple sclerosis. Nat Rev Neurosci. 2024;25(7):493–513. - PubMed
-
- Bachmann H, Vandemoortele B, Vermeirssen V, Carrette E, Vonck K, Boon P, Raedt R, et al. Vagus nerve stimulation enhances remyelination and decreases innate neuroinflammation in lysolecithin-induced demyelination. Brain Stimul. 2024;17(3):575–87. - PubMed
-
- McMurran CE, Zhao C, Franklin RJM. Toxin-based models to investigate demyelination and remyelination. Methods Mol Biol. 2019;1936:377–96. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

