Maternal stress during pregnancy alters circulating small extracellular vesicles and enhances their targeting to the placenta and fetus
- PMID: 39342314
- PMCID: PMC11438166
- DOI: 10.1186/s40659-024-00548-4
Maternal stress during pregnancy alters circulating small extracellular vesicles and enhances their targeting to the placenta and fetus
Abstract
Background: Maternal psychological distress during pregnancy can negatively impact fetal development, resulting in long-lasting consequences for the offspring. These effects show a sex bias. The mechanisms whereby prenatal stress induces functional and/or structural changes in the placental-fetal unit remain poorly understood. Maternal circulating small extracellular vesicles (sEVs) are good candidates to act as "stress signals" in mother-to-fetus communication. Using a repetitive restraint-based rat model of prenatal stress, we examined circulating maternal sEVs under stress conditions and tested whether they could target placental-fetal tissues.
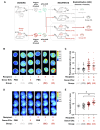
Results: Our mild chronic maternal stress during pregnancy paradigm induced anhedonic-like behavior in pregnant dams and led to intrauterine growth restriction (IUGR), particularly in male fetuses and placentas. The concentration and cargo of maternal circulating sEVs changed under stress conditions. Specifically, there was a significant reduction in neuron-enriched proteins and a significant increase in astrocyte-enriched proteins in blood-borne sEVs from stressed dams. To study the effect of repetitive restraint stress on the biodistribution of maternal circulating sEVs in the fetoplacental unit, sEVs from pregnant dams exposed to stress or control protocol were labeled with DiR fluorescent die and injected into pregnant females previously exposed to control or stress protocol. Remarkably, maternal circulating sEVs target placental/fetal tissues and, under stress conditions, fetal tissues are more receptive to sEVs.
Conclusion: Our results suggest that maternal circulating sEVs can act as novel mediators/modulators of mother-to-fetus stress communication. Further studies are needed to identify placental/fetal cellular targets of maternal sEVs and characterize their contribution to stress-induced sex-specific placental and fetal changes.
Keywords: Biodistribution; Exosomes; Fetus; Placenta; Prenatal stress; Restraint; Sex-bias.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
MeSH terms
Grants and funding
- Fondecyt 1141015/Agencia Nacional de Investigación y Desarrollo
- Fondecyt 1201851/Agencia Nacional de Investigación y Desarrollo
- Fondecyt 1211384/Agencia Nacional de Investigación y Desarrollo
- Fondecyt 1230932/Agencia Nacional de Investigación y Desarrollo
- Fondef ID19I10116/Agencia Nacional de Investigación y Desarrollo
- COVID0706/Agencia Nacional de Investigación y Desarrollo
- FB210024/Agencia Nacional de Investigación y Desarrollo
- Fondecyt 11220601/Agencia Nacional de Investigación y Desarrollo
- Fondecyt 1200693/Agencia Nacional de Investigación y Desarrollo
- Fondecyt 1240604/Agencia Nacional de Investigación y Desarrollo
- HHSN275201300006C/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

