Neoadjuvant chemotherapy induces phenotypic mast cell changes in high grade serous ovarian cancer
- PMID: 39342316
- PMCID: PMC11438021
- DOI: 10.1186/s13048-024-01516-y
Neoadjuvant chemotherapy induces phenotypic mast cell changes in high grade serous ovarian cancer
Abstract
Background: High grade serous ovarian cancer (HGSOC) is the most lethal gynecologic malignancy in which patients have still yet to respond meaningfully to clinically available immunotherapies. Hence, novel immune targets are urgently needed. Our past work has identified that mast cells are significantly upregulated at the mRNA level in HGSOC patient tumors following neoadjuvant chemotherapy (NACT) exposure. Therefore, in this current investigation we sought to characterize intratumoral mast cell phenotypic changes as a result of NACT exposure and determine how these adaptations are associated with patient clinical outcomes.
Methods: Hematologic immunohistochemistry was employed to determine mast cell levels in 36 matched pre- and post-NACT HGSOC patient tumors. Fluorescent Immunohistochemistry was utilized to identify Tryptase+(carboxypeptidase A3 (CPA3) + mast cells as well as histamine levels in 29 and 20, respectively, matched pre- and post-NACT HGSOC patient tumors. Finally, human immortalized mast cells, LUVA were stimulated with carboplatin and paclitaxel and genomic changes were analyzed by quantitative PCR.
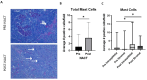
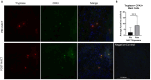
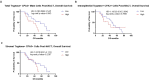
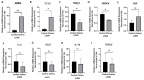
Results: Hematologic labeled intratumoral mast cells were significantly upregulated in the intraepithelial and stromal regions of the tumor, post-NACT. Lower levels of pre-NACT mast cells were significantly associated with an improved progression-free survival (PFS). Histamine, a marker of mast cell degranulation was similarly upregulated in post-NACT exposed tumors. Through the characterization of mast cell specific proteases Tryptase and CPA3, it was found that Tryptase+/ CPA3 + mast cells were significantly upregulated both in the intraepithelial and stromal compartments of the tumor, while Tryptase + cells were significantly upregulated in the stromal regions of the tumor. Lower post-NACT treated levels with Tryptase+/ CPA3 + cells were significantly associated with improved overall survival (OS) and PFS while higher Tryptase + mast cells were associated with improved OS. Finally, following chemotherapy exposure mast cell activating factors AREG and CCL2 were significantly upregulated while TGFB1, an inhibitor of mast cell activation was downregulated in LUVA cells.
Conclusions: Enhanced mast cell numbers, as well as activation and degranulation are a consequence of NACT exposure. Post-NACT mast cells displayed differing associations with survival outcomes that was dependent upon granule classification. Ultimately, mast cells represent a clinically relevant putative HGSOC immune target.
Keywords: High grade serous ovarian cancer; Mast cells; Neoadjuvant chemotherapy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Molecular Response to Neoadjuvant Chemotherapy in High-Grade Serous Ovarian Carcinoma.Mol Cancer Res. 2018 May;16(5):813-824. doi: 10.1158/1541-7786.MCR-17-0594. Epub 2018 Mar 9. Mol Cancer Res. 2018. PMID: 29523763 Free PMC article.
-
Identification of high-grade serous ovarian cancer miRNA species associated with survival and drug response in patients receiving neoadjuvant chemotherapy: a retrospective longitudinal analysis using matched tumor biopsies.Ann Oncol. 2016 Apr;27(4):625-34. doi: 10.1093/annonc/mdw007. Epub 2016 Jan 17. Ann Oncol. 2016. PMID: 26782955
-
Clonal Evolution of TP53 c.375+1G>A Mutation in Pre- and Post- Neo-Adjuvant Chemotherapy (NACT) Tumor Samples in High-Grade Serous Ovarian Cancer (HGSOC).Cells. 2019 Oct 1;8(10):1186. doi: 10.3390/cells8101186. Cells. 2019. PMID: 31581548 Free PMC article.
-
Uptake and Outcomes of Neoadjuvant Chemotherapy Among US Patients With Less Common Epithelial Ovarian Carcinomas.JAMA Netw Open. 2023 Jun 1;6(6):e2318602. doi: 10.1001/jamanetworkopen.2023.18602. JAMA Netw Open. 2023. PMID: 37326992 Free PMC article.
-
Past failures and new horizons: the nuances of tertiary lymphoid structures in high-grade serous ovarian cancer may contribute to immunotherapy effectiveness.J Immunother Cancer. 2025 Apr 17;13(4):e011670. doi: 10.1136/jitc-2025-011670. J Immunother Cancer. 2025. PMID: 40246584 Free PMC article. Review.
References
-
- Luvero D, Plotti F, Aloisia A, Montera R, Terranova C et al. Carlo De Cicco Nardone,. Ovarian cancer relapse: From the latest scientific evidence to the best practice. Crit Rev Oncol Hematol. 2019;140:28–38. - PubMed
-
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–13. - PubMed
-
- James NE, Woodman M, De La Cruz P, Eurich K, Ozsoy MA, Schorl C, et al. Adaptive transcriptomic and immune infiltrate responses in the tumor immune microenvironment following neoadjuvant chemotherapy in high grade serous ovarian cancer reveal novel prognostic associations and activation of pro-tumorigenic pathways. Front Immunol. 2022;13:965331. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

