Akkermansia muciniphila improve cognitive dysfunction by regulating BDNF and serotonin pathway in gut-liver-brain axis
- PMID: 39342324
- PMCID: PMC11438137
- DOI: 10.1186/s40168-024-01924-8
Akkermansia muciniphila improve cognitive dysfunction by regulating BDNF and serotonin pathway in gut-liver-brain axis
Erratum in
-
Correction: Akkermansia muciniphila improve cognitive dysfunction by regulating BDNF and serotonin pathway in gut-liver-brain axis.Microbiome. 2025 Nov 4;13(1):226. doi: 10.1186/s40168-025-02247-y. Microbiome. 2025. PMID: 41189004 Free PMC article. No abstract available.
Abstract
Backrground: Akkermansia muciniphila, a next-generation probiotic, is known as a cornerstone regulating the gut-organ axis in various diseases, but the underlying mechanism remains poorly understood. Here, we revealed the neuronal and antifibrotic effects of A. muciniphila on the gut-liver-brain axis in liver injury.
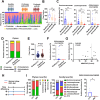
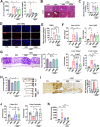
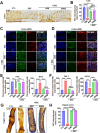
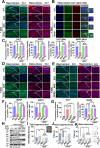
Results: To investigate neurologic dysfunction and characteristic gut microbiotas, we performed a cirrhosis cohort (154 patients with or without hepatic encephalopathy) and a community cognition cohort (80 participants in one region for three years) and validated the existence of cognitive impairment in a 3,5-diethoxycarbonyl-1,4-dihydrocollidine-induced hepatic injury mouse model. The effects of the candidate strain on cognition were evaluated in animal models of liver injury. The expression of brain-derived neurotrophic factor (BDNF) and serotonin receptors was accessed in patients with fibrosis (100 patients) according to the fibrosis grade and hepatic venous pressure gradient. The proportion of A. muciniphila decreased in populations with hepatic encephalopathy and cognitive dysfunction. Tissue staining techniques confirmed gut-liver-brain damage in liver injury, with drastic expression of BDNF and serotonin in the gut and brain. The administration of A. muciniphila significantly reduced tissue damage and improved cognitive dysfunction and the expression of BDNF and serotonin. Isolated vagus nerve staining showed a recovery of serotonin expression without affecting the dopamine pathway. Conversely, in liver tissue, the inhibition of injury through the suppression of serotonin receptor (5-hydroxytryptamine 2A and 2B) expression was confirmed. The severity of liver injury was correlated with the abundance of serotonin, BDNF, and A. muciniphila.
Conclusions: A. muciniphila, a next-generation probiotic, is a therapeutic candidate for alleviating the symptoms of liver fibrosis and cognitive impairment.
Keywords: Akkermansia muciniphila; BDNF; Cognitive impairment; Gut-organ axis; Liver injury; Serotonin.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Shawcross DL, Thabut D, Amodio P. Ammonia - an enduring foe - What evaluating whole body ammonia metabolism can teach us about cirrhosis and therapies treating hepatic encephalopathy. J Hepatol. 2023;79(2):266–8. - PubMed
-
- Haeussinger D, Dhiman RK, Felipo V, Görg B, Jalan R, Kircheis G, et al. Hepatic encephalopathy Nature Reviews Disease Primers. 2022;8(1):43. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

