LYP regulates SLP76 and other adaptor proteins in T cells
- PMID: 39342392
- PMCID: PMC11438317
- DOI: 10.1186/s40659-024-00536-8
LYP regulates SLP76 and other adaptor proteins in T cells
Abstract
Background: The LYP tyrosine phosphatase presents a SNP (1858C > T) that increases the risk of developing autoimmune diseases such as type I diabetes and arthritis. It remains unclear how this SNP affects LYP function and promotes the development of these diseases. The scarce information about LYP substrates is in part responsible for the poor understanding of LYP function.
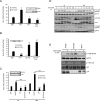
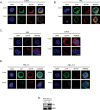
Results: In this study, we identify in T lymphocytes several adaptor proteins as potential substrates targeted by LYP, including FYB, SLP-76, HS-1, Vav, SKAP1 and SKAP2. We also show that LYP co-localizes with SLP76 in microclusters, upon TCR engagement.
Conclusions: These data indicate that LYP may modulate T cell activation by dephosphorylating several adaptor proteins, such as FYB, SLP-76, HS-1, Vav, SKAP1 and SKAP2 upon TCR engagement.
Keywords: FYB; LYP (lymphoid phosphatase); Protein tyrosine phosphatase (PTP); SKAP2; SLP76; T-cell; T-cell receptor (TCR).
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
The lymphoid protein tyrosine phosphatase Lyp interacts with the adaptor molecule Grb2 and functions as a negative regulator of T-cell activation.Exp Hematol. 2002 Mar;30(3):237-44. doi: 10.1016/s0301-472x(01)00794-9. Exp Hematol. 2002. PMID: 11882361
-
Receptor-stimulated oxidation of SHP-2 promotes T-cell adhesion through SLP-76-ADAP.EMBO J. 2005 Jul 6;24(13):2331-41. doi: 10.1038/sj.emboj.7600706. Epub 2005 Jun 2. EMBO J. 2005. PMID: 15933714 Free PMC article.
-
ADAP is an upstream regulator that precedes SLP-76 at sites of TCR engagement and stabilizes signaling microclusters.J Cell Sci. 2018 Nov 8;131(21):jcs215517. doi: 10.1242/jcs.215517. J Cell Sci. 2018. PMID: 30305305 Free PMC article.
-
The Role of Adaptor Proteins in the Biology of Natural Killer T (NKT) Cells.Front Immunol. 2019 Jun 25;10:1449. doi: 10.3389/fimmu.2019.01449. eCollection 2019. Front Immunol. 2019. PMID: 31293596 Free PMC article. Review.
-
The Multiple Roles of the Cytosolic Adapter Proteins ADAP, SKAP1 and SKAP2 for TCR/CD3 -Mediated Signaling Events.Front Immunol. 2021 Jul 6;12:703534. doi: 10.3389/fimmu.2021.703534. eCollection 2021. Front Immunol. 2021. PMID: 34295339 Free PMC article. Review.
References
-
- Vang T, Miletic A V, Arimura Y, Tautz L, Rickert RC, Mustelin T. Protein tyrosine phosphatases in autoimmunity. 2008; 26:29–55. 10.1146/annurev.immunol.26.021607.090418 - PubMed
-
- Badour K, Zhang J, Shi F, Leng Y, Collins M, Siminovitch KA. Fyn and PTP-PEST-mediated regulation of Wiskott-Aldrich Syndrome Protein (WASp) tyrosine phosphorylation is required for coupling T cell antigen receptor engagement to WASp effector function and T cell activation. J Exp Med. 2004;199(1):99–111. 10.1084/jem.20030976 - PMC - PubMed
-
- Marcos T, Ruiz-Martín V, De La Puerta ML, Trinidad AG, Rodríguez MDC, De La Fuente MA, et al. Proline-serine-threonine phosphatase interacting protein 1 inhibition of T-cell receptor signaling depends on its SH3 domain. FEBS J. 2014;281(17):3844–54. 10.1111/febs.12912 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

