GTSE1-driven ZEB1 stabilization promotes pulmonary fibrosis through the epithelial-to-mesenchymal transition
- PMID: 39342428
- PMCID: PMC11573610
- DOI: 10.1016/j.ymthe.2024.09.029
GTSE1-driven ZEB1 stabilization promotes pulmonary fibrosis through the epithelial-to-mesenchymal transition
Abstract
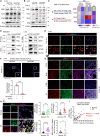
G2 and S phase-expressed protein 1 (GTSE1) has been implicated in the development of pulmonary fibrosis (PF); however, its biological function, molecular mechanism, and potential clinical implications remain unknown. Here, we explored the genomic data of patients with idiopathic PF (IPF) and found that GTSE1 expression is elevated in their lung tissues, but rarely expressed in normal lung tissues. Thus, we explored the biological role and downstream events of GTSE1 using IPF patient tissues and PF mouse models. The comprehensive bioinformatics analyses suggested that the increase of GTSE1 in IPF is linked to the enhanced gene signature for the epithelial-to-mesenchymal transition (EMT), leading us to investigate the potential interaction between GTSE1 and EMT transcription factors. GTSE1 preferentially binds to the less stable form of zinc-finger E-box-binding homeobox 1 (ZEB1), the unphosphorylated form at Ser585, inhibiting ZEB1 degradation. Consistently, the ZEB1 protein level in IPF patient and PF mouse tissues correlates with the GTSE1 protein level and the amount of collagen accumulation, representing fibrosis severity. Collectively, our findings highlight the GTSE1-ZEB1 axis as a novel driver of the pathological EMT characteristic during PF development and progression, supporting further investigation into GTSE1-targeting approaches for PF treatment.
Keywords: GTSE1; IPF; RNA therapeutics; ZEB1; epithelial mesenchymal transition; lipid nanoparticles; protein stability; pulmonary fibrosis; ubiquitin degradation.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures







References
-
- Richeldi L., Du Bois R.M., Raghu G., Azuma A., Brown K.K., Costabel U., Cottin V., Flaherty K.R., Hansell D.M., Inoue Y., et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014;370:2071–2082. - PubMed
-
- Richeldi L., Azuma A., Cottin V., Hesslinger C., Stowasser S., Valenzuela C., Wijsenbeek M.S., Zoz D.F., Voss F., Maher T.M., 1305-0013 Trial Investigators Trial of a preferential phosphodiesterase 4B inhibitor for idiopathic pulmonary fibrosis. N. Engl. J. Med. 2022;386:2178–2187. - PubMed
-
- Noble P.W., Albera C., Bradford W.Z., Costabel U., Du Bois R.M., Fagan E.A., Fishman R.S., Glaspole I., Glassberg M.K., Lancaster L., et al. Pirfenidone for idiopathic pulmonary fibrosis: analysis of pooled data from three multinational phase 3 trials. Eur. Respir. J. 2016;47:243–253. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

