Design, Synthesis and Characterization of Mn(II)Cysteine-Tyrosine Dithiocarbamate Complex for against the Cancer on MCF-7 Breast Cancer Cell Line
- PMID: 39342604
- PMCID: PMC11700321
- DOI: 10.31557/APJCP.2024.25.9.3251
Design, Synthesis and Characterization of Mn(II)Cysteine-Tyrosine Dithiocarbamate Complex for against the Cancer on MCF-7 Breast Cancer Cell Line
Abstract
Objective: Breast cancer is the most frequently diagnosed cancer and the second cause of death worldwide. The drug often used for chemotherapy is cisplatin. However, the drug cisplatin has a number of problems, including lack of selectivity, undesirable side effects, resistance, and toxicity in the body. So research is carried out on new drug compounds with low toxicity by designing in silico with molecular docking.
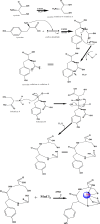
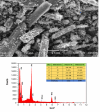
Methods: Mn(II) Cysteine-Tyrosine dithiocarbamate is a new complex molecule whose research involves several steps, such as in-silico molecular docking testing with target proteins, ADMET then synthesis, characterization and in-vitro MCF-7 cells for anticancer drugs. The synthesis process involves the reaction of manganese metal with tyrosine, cysteine, CS2 and KOH. Characterization tests have been carried out including FT-IR spectroscopy, SEM-EDS, UV Vis, conductivity, melting point and XRD.
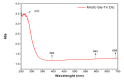
Result: Confirm the structure of the compound using UV Vis, obtained orbitals π to π* and n to π* in the group N = C = S is represented by the absorption at 400 nm and 600 nm, FT-IR with the results obtained by the functional groups O-H, N-H, C =N and C=S. In vitro test results showed morphological changes (apoptosis) in MCF-7 cancer cells starting from 250 μg/mL and an IC50 value of 416.90 µg/mL. Molecular docking studies of the Mn(II)Cysteine-Tyrosine dithiocarbamate complex were identified with 4,4',4''-[(2R)-butane-1,1,2-triyl]triphenol - Estrogen α which showed an active site with amino acid residues GLU323, GLU385, VAL446, ILE514, TRP360, LYS449, MET388, MET357, PHE445, VAL392 and ILE389. Hydrophobic and hydrophobic bonds are seen in Mn(II)Cysteine-Tyrosine dithiocarbamate - Estrogen α has a bond energy of -77.5372 kJ/mol.
Conclusion: Despite having a high H-bond interaction intensity, the chemical does not have a powerful enough anticancer impact. Despite the produced compound's low bioactivity, this study should offer important new understandings into how molecular structure affects anticancer activity.
Keywords: Complex; IC50; MCF-7 cell lines; Mn(II)Cysteine-Tyrosine dithiocarbamate; Molecular docking.
Conflict of interest statement
The authors declare that none of the work reported in this study could have been influenced by any known competing financial interests or personal relationships.
Figures






References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. - PubMed
-
- Zhang W, Yao D, Wei Y, Tang J, Bian H-D, Huang F-P, et al. Synthesis, characterization, DNA/protein interaction and cytotoxicity studies of cu(ii) and co(ii) complexes derived from dipyridyl triazole ligands. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2016;163:28–44. - PubMed
-
- Romani AMP. Cisplatin in cancer treatment. Biochem Pharmacol. 2022;206:115323. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

