Finerenone Improves Outcomes in Patients With Heart Failure With Mildly Reduced or Preserved Ejection Fraction Irrespective of Age: A Prespecified Analysis of FINEARTS-HF
- PMID: 39342655
- PMCID: PMC11573060
- DOI: 10.1161/CIRCHEARTFAILURE.124.012437
Finerenone Improves Outcomes in Patients With Heart Failure With Mildly Reduced or Preserved Ejection Fraction Irrespective of Age: A Prespecified Analysis of FINEARTS-HF
Abstract
Background: Finerenone improves outcomes in patients with heart failure and mildly reduced or preserved ejection fraction. It is important to understand the efficacy and safety of finerenone in these patients according to age.
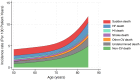
Methods: The aim of this analysis was to evaluate the interaction between age and the efficacy and safety of finerenone in the FINEARTS-HF trial (Finerenone Trial to Investigate Efficacy and Safety Compared to Placebo in Patients With Heart Failure). A total of 6001 patients aged 40 to 97 years were stratified by quartile (Q1-Q4) of baseline age: Q1, 40 to 66 years (n=1581); Q2, 67 to 73 years (n=1587); Q3, 74 to 79 years (n=1421); and Q4, ≥80 years (n=1412). FINEARTS-HF evaluated the impact of age on the efficacy of finerenone with respect to the primary composite outcome of cardiovascular death and total (first and recurrent) heart failure events, including heart failure hospitalization or urgent heart failure event, along with secondary efficacy and safety outcomes.
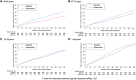
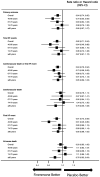
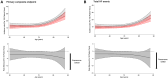
Results: The incidence of primary outcomes increased with age. Finerenone reduced the risk of the primary outcome consistently across all age categories: rate ratio in Q1, 0.70 (95% CI, 0.53-0.92); Q2, 0.83 (95% CI, 0.64-1.07); Q3, 0.98 (95% CI, 0.76-1.26); and Q4, 0.85 (95% CI, 0.67-1.07); Pinteraction=0.27. Similarly, a consistent effect was observed for the components of the primary outcome. The mean increase in Kansas City Cardiomyopathy Questionnaire-total symptom score from baseline to 12 months was greater with finerenone than placebo, with a consistent effect across all age categories: mean placebo-corrected change in Q1, 2.87 (95% CI, 1.09-4.66); Q2, 1.24 (95% CI, -0.59 to 3.07); Q3, 0.94 (-0.98 to 2.86); and Q4, 1.24 (-0.90 to 3.38); Pinteraction=0.50. Adverse events were similar across all age categories. The odds of experiencing hypotension, elevated creatinine, or hyperkalemia (increased) or hypokalemia (decreased) related to finerenone did not differ by age.
Conclusions: In the FINEARTS-HF trial, finerenone reduced the primary outcome and components of the primary outcome and improved symptoms across a wide age spectrum. In addition, finerenone was safe and well-tolerated, irrespective of age.
Registration: URL: https://www.clinicaltrials.gov; Unique identifiers: NCT04435626 and EudraCT 2020-000306-29.
Keywords: age; finerenone; heart failure; hospitalization.
Conflict of interest statement
Dr Chimura received research grants and personal fees from Otsuka Pharma, Daiwa Foundation, and the Japan Research Foundation for Clinical Pharmacology. Dr Petrie reports, outside of the submitted work, grants or contracts from Boehringer Ingelheim, Roche, SQ Innovations, AstraZeneca, Novartis, Novo Nordisk, Medtronic, Boston Scientific, and Pharmacosmos; consulting fees from Akero, Applied Therapeutics, Amgen, AnaCardio, Biosensors, Boehringer Ingelheim, Novartis, AstraZeneca, Novo Nordisk, Abbvie, Bayer, Horizon Therapeutics, Foundry, Takeda, Cardiorentis, Pharmacosmos, Siemens, Eli Lilly, Vifor, New Amsterdam, Moderna, Teikoku, LIB Therapeutics, and 3R Lifesciences; and is Director of Global Clinical Trials Partners. Dr Schou reports other from Novo Nordisk, Novartis, AstraZeneca, and Boehringer outside the submitted work and reports personal fees from Bayer during the conduct of the study and personal fees from Alleviant, AstraZeneca, Boehringer Ingelheim, Edwards Lifesciences, Janssen, Novartis, Novo Nordisk, and Rivus outside the study. Dr Martinez reports personal fees from Bayer, during the conduct of the study. Dr Claggett has received personal consulting fees from Alnylam, Bristol Myers Squibb (BMS), Cardior, Cardurion, Corvia, CVRx, Eli Lilly, Intellia, and Rocket and has served on a data safety monitoring board for Novo Nordisk. Dr Desai has received institutional research grants (to Brigham and Women’s Hospital) from Abbott, Alnylam, AstraZeneca, Bayer, Novartis, and Pfizer, as well as personal consulting fees from Abbott, Alnylam, AstraZeneca, Bayer, Biofourmis, Boston Scientific, Medpace, Medtronic, Merck, Novartis, Parexel, Porter Health, Regeneron, River2Renal, Roche, Veristat, Verily, and Zydus. Drs Kolkhof, Lage, and Rohwedder and K. Mueller are employees of Bayer. Dr Viswanathan is a full-time employee of Bayer Pharmaceuticals. Dr Lam has received research support from NovoNordisk and Roche Diagnostics; has received consulting fees from Alleviant Medical, Allysta Pharma, AnaCardio AB, Applied Therapeutics, AstraZeneca, Bayer, Biopeutics, Boehringer Ingelheim, Boston Scientific, BMS, CardioRenal, CPC Clinical Research, Eli Lilly, Impulse Dynamics, Intellia Therapeutics, Ionis Pharmaceutical, Janssen Research and Development LLC, Medscape/WebMD Global LLC, Merck, Novartis, Novo Nordisk, Prosciento Inc, Quidel Corporation, Radcliffe Group Ltd, Recardio Inc, ReCor Medical, Roche Diagnostics, Sanofi, Siemens Healthcare Diagnostics, and Us2.ai; and is a cofounder and nonexecutive director of Us2.ai. Dr Senni has served on advisory boards, consultancy and honoraria for Novartis, Abbott, Merck, Vifor, AstraZeneca, Cardurion, Novo Nordisk, Bayer, and Boehringer Ingelheim. Dr Shah has received research grants from the National Institutes of Health (NIH; U54 HL160273, X01 HL169712, R01 HL140731, and R01 HL149423), the American Heart Association (24SFRNPCN1291224), AstraZeneca, Corvia, and Pfizer; and received consulting fees from Abbott, Alleviant, AstraZeneca, Amgen, Aria CV, Axon Therapies, Bayer, Boehringer Ingelheim, Boston Scientific, BMS, Cyclerion, Cytokinetics, Edwards Lifesciences, Eidos, Imara, Impulse Dynamics, Intellia, Ionis, Lilly, Merck, MyoKardia, Novartis, Novo Nordisk, Pfizer, Prothena, Regeneron, Rivus, Sardocor, Shifamed, Tenax, Tenaya, and Ultromics. Dr Voors’ employer received consultancy fees and/or research support from Adrenomed, Anacardio, AstraZeneca, Bayer AG, BMS, Boehringer Ingelheim, Corteria, EliLilly, Merck, Moderna, Novartis, Novo Nordisk, Roche Diagnostics, and SalubrisBio. Dr Zannad reports personal fees from 89Bio, Abbott, Acceleron, Applied Therapeutics, Bayer, Betagenon, Boehringer, BMS, CVRx, Cambrian, Cardior, Cereno Pharmaceutical, Cellprothera, CEVA, Inventiva, KBP, Merck, NovoNordisk, Owkin, Otsuka, Roche Diagnostics, Northsea, and USa2, having stock options at G3Pharmaceutical and equities at Cereno, Cardiorenal, and Eshmoun Clinical Research, and being the founder of Cardiovascular Clinical Trialists. Dr Pitt is a consultant for Bayer, Astra Zeneca, Boehringer Ingelheim, Lexicon, BMS, KBP Biosciences, Sarfez Pharmaceuticals, Pharmaceuticals, SQinnovations, G3 Pharmaceuticals, Sea Star Medical, Vifor, Prointel, and Brainstorm Medical; stock/stock options for KBP Biosciences, Sarfez Pharmaceuticals, Pharmaceuticals, SQinnovations, Sea Star Medical, Vifor, Prointel, and Brainstorm Medical; and US Patent 9931412-site specific delivery of eplerenone to the myocardium and US Patent pending 63/045,783 Histone modulating agents for the prevention and treatment of organ failure. Dr Vaduganathan has received research grant support, served on advisory boards, or had speaker engagements with American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, BMS, Boehringer Ingelheim, Chiesi, Cytokinetics, Fresenius Medical Care, Idorsia Pharmaceuticals, Lexicon Pharmaceuticals, Merck, Milestone Pharmaceuticals, Novartis, Novo Nordisk, Pharmacosmos, Relypsa, Roche Diagnostics, Sanofi, and Tricog Health; and participates on clinical trial committees for studies sponsored by AstraZeneca, Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics. Dr Jhund reports speakers’ fees from AstraZeneca, Novartis, Alkem Metabolics, ProAdWise Communications, and Sun Pharmaceuticals; advisory board fees from AstraZeneca, Boehringer Ingelheim, and Novartis; and research funding from AstraZeneca, Boehringer Ingelheim, Analog Devices Inc, and Roche Diagnostics. Dr Jhund’s employer, the University of Glasgow, has been remunerated for clinical trial work from AstraZeneca, Bayer AG, Novartis, and Novo Nordisk; and is the director of GCTP Ltd. Dr Solomon has received research grants from Alexion, Alnylam, AstraZeneca, Bellerophon, Bayer, BMS, Boston Scientific, Cytokinetics, Edgewise, Eidos, Gossamer, GSK, Ionis, Lilly, MyoKardia, the NIH/National Heart, Lung, and Blood Institute (NHLBI), Novartis, NovoNordisk, Respicardia, Sanofi Pasteur, Theracos, and Us2.ai; and has consulted for Abbott, Action, Akros, Alexion, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boeringer Ingelheim, BMS, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi-Sankyo, GlaxoSmithKline (GSK), Lilly, Merck, Myokardia, Novartis, Roche, Theracos, Quantum Genomics, Janssen, Cardiac Dimensions, Tenaya, Sanofi Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, American Regent, Sarepta, Lexicon, Anacardio, Akros, and Valo. Dr McMurray reports payments through Glasgow University from work on clinical trials, consulting, and grants from Amgen, AstraZeneca, Bayer, Cardurion, Cytokinetics, GSK, and Novartis, the British Heart Foundation, the NIH/NHLBI, Boehringer Ingelheim, SQ Innovations, and Catalyze Group; personal consultancy fees from Alynylam Pharmaceuticals, Amgen, AnaCardio, AstraZeneca, Bayer, Berlin Cures, BMS, Cardurion, Cytokinetics, Ionis Pharmaceuticals, Novartis, Regeneron Pharmaceuticals, and River 2 Renal Corp; personal lecture fees from Abbott, Alkem Metabolics, Astra Zeneca, Blue Ocean Scientific Solutions Ltd, Boehringer Ingelheim, Canadian Medical and Surgical Knowledge, Emcure Pharmaceuticals Ltd, Eris Lifesciences, European Academy of CME, Hikma Pharmaceuticals, Imagica Health, Intas Pharmaceuticals, JB Chemicals and Pharmaceuticals Ltd, Lupin Pharmaceuticals, Medscape/Heart.Org, ProAdWise Communications, Radcliffe Cardiology, Sun Pharmaceuticals, The Corpus, Translation Research Group, and Translational Medicine Academy; and Data Safety Monitoring Board for WIRB-Copernicus Group Clinical Inc. He is a director of Global Clinical Trial Partners Ltd. All other authors report no conflicts.
Figures
References
-
- Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14:591–602. doi: 10.1038/nrcardio.2017.65 - PubMed
-
- Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895–e1032. doi: 10.1161/CIR.0000000000001063 - PubMed
-
- Yang M, Kondo T, Anand IS, de Boer RA, Campbell RT, Køber L, Lam CSP, Maggioni AP, Martinez FA, O’Meara E, et al. Clinical characteristics and outcomes of patients aged 80 years and over with heart failure: need for better treatment. Eur J Heart Fail. 2024. doi: 10.1002/ejhf.3417 - PubMed
-
- Miró O, Gil V, Martín-Sánchez FJ, Jacob J, Herrero P, Alquézar A, Llauger L, Aguiló S, Martínez G, Ríos J, et al. Short-term outcomes of heart failure patients with reduced and preserved ejection fraction after acute decompensation according to the final destination after emergency department care. Clin Res Cardiol. 2018;107:698–710. doi: 10.1007/s00392-018-1237-z - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

