A fluorescence-based assay for measuring polyamine biosynthesis aminopropyl transferase-mediated catalysis
- PMID: 39342998
- PMCID: PMC11541840
- DOI: 10.1016/j.jbc.2024.107832
A fluorescence-based assay for measuring polyamine biosynthesis aminopropyl transferase-mediated catalysis
Abstract
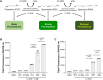
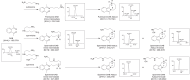
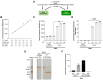
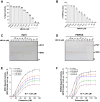
Polyamines are polycationic molecules that are crucial in a wide array of cellular functions. Their biosynthesis is mediated by aminopropyl transferases (APTs), which are promising targets for antimicrobial, antineoplastic, and antineurodegenerative therapies. A major limitation in studying APT enzymes, however, is the lack of high-throughput assays to measure their activity. We have developed the first fluorescence-based assay, diacetyl benzene (DAB)-APT, for the measurement of APT activity using 1,2-DAB, which forms fluorescent conjugates with putrescine, spermidine, and spermine, with fluorescence intensity increasing with the carbon chain length. The assay has been validated using APT enzymes from Saccharomyces cerevisiae and Plasmodium falciparum, and the data further validated by mass spectrometry and TLC. Using mass spectrometry analysis, the structures of the fluorescent putrescine, spermidine, and spermine 1,2-DAB adducts were determined to be substituted 1,3-dimethyl isoindoles. The DAB-APT assay is optimized for high-throughput screening, facilitating the evaluation of large chemical libraries. Given the critical roles of APTs in infectious diseases, oncology, and neurobiology, the DAB-APT assay offers a powerful tool with broad applicability, poised to drive advancements in research and drug discovery.
Keywords: 1,2-diacetyl benzene; Babesia; Plasmodium; aminopropyl transferases; drug discovery; enzyme activity; fluorescence assay; inhibition; isoindole; kinetics; polyamines; putrescine; spermidine; spermine; yeast.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest C. B. M. is listed on a provisional patent application on the use of DAB-APT assay and its use to discover inhibitors of APT enzymes. The other authors declare that they have no conflicts of interest with the contents of this article.
Figures




References
-
- Cohen S.S. Vol. 1. Oxford University Press; 1998. A guide to the polyamines; p. 624.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

