Chemical genetic analysis of enoxolone inhibition of Clostridioides difficile toxin production reveals adenine deaminase and ATP synthase as antivirulence targets
- PMID: 39343002
- PMCID: PMC11566853
- DOI: 10.1016/j.jbc.2024.107839
Chemical genetic analysis of enoxolone inhibition of Clostridioides difficile toxin production reveals adenine deaminase and ATP synthase as antivirulence targets
Abstract
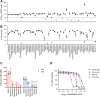
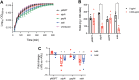
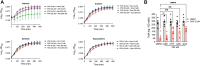
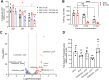
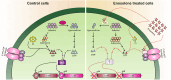
Toxins TcdA and TcdB are the main virulence factors of Clostridioides difficile, a leading cause of hospital-acquired diarrhea. Despite their importance, there is a significant knowledge gap of druggable targets for inhibiting toxin production. To address this, we screened nonantibiotic phytochemicals to identify potential chemical genetic probes to discover antivirulence drug targets. This led to the identification of 18β-glycyrrhetinic acid (enoxolone), a licorice metabolite, as an inhibitor of TcdA and TcdB biosynthesis. Using affinity-based proteomics, potential targets were identified as ATP synthase subunit alpha (AtpA) and adenine deaminase (Ade, which catalyzes conversion of adenine to hypoxanthine in the purine salvage pathway). To validate these targets, a multifaceted approach was adopted. Gene silencing of ade and atpA inhibited toxin biosynthesis, while surface plasmon resonance and isothermal titration calorimetry molecular interaction analyses revealed direct binding of enoxolone to Ade. Metabolomics demonstrated enoxolone induced the accumulation of adenosine, while depleting hypoxanthine and ATP in C. difficile. Transcriptomics further revealed enoxolone dysregulated phosphate uptake genes, which correlated with reduced cellular phosphate levels. These findings suggest that enoxolone's cellular action is multitargeted. Accordingly, supplementation with both hypoxanthine and triethyl phosphate, a phosphate source, was required to fully restore toxin production in the presence of enoxolone. In conclusion, through the characterization of enoxolone, we identified promising antivirulence targets that interfere with nucleotide salvage and ATP synthesis, which may also block toxin biosynthesis.
Keywords: ATP synthase; adenine deaminase; phosphate metabolism; purine metabolism; sporulation; toxins.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

