Plant sesquiterpene lactones
- PMID: 39343024
- PMCID: PMC11449222
- DOI: 10.1098/rstb.2023.0350
Plant sesquiterpene lactones
Abstract
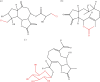
Sesquiterpene lactones (STLs) are a prominent group of plant secondary metabolites predominantly found in the Asteraceae family and have multiple ecological roles and medicinal applications. This review describes the evolutionary and ecological significance of STLs, highlighting their roles in plant defence mechanisms against herbivory and as phytotoxins, alongside their function as environmental signalling molecules. We also cover the substantial role of STLs in medicine and their mode of action in health and disease. We discuss the biosynthetic pathways and the various modifications that make STLs one of the most diverse groups of metabolites. Finally, we discuss methods for identifying and predicting STL biosynthesis pathways. This article is part of the theme issue 'The evolution of plant metabolism'.
Keywords: bioinformatics; biosynthesis; medicinal; plants; sesquiterpene; specialized metabolism.
Conflict of interest statement
We declare we have no competing interests.
Figures


References
-
- Picman AK. 1986. Biological activities of sesquiterpene lactones. Biochem. Syst. Ecol. 14 , 255–281. ( 10.1016/0305-1978(86)90101-8) - DOI
-
- Schmidt TJ. 2006. Structure-activity relationships of sesquiterpene lactones. In Studies in natural products chemistry (ed. Rahman Atta-ur-), pp. 309–392. Amsterdam, The Netherlands: Elsevier. ( 10.1016/S1572-5995(06)80030-X). See https://www.sciencedirect.com/science/article/pii/S157259950680030X. - DOI
-
- Rodriguez E, Towers GHN, Mitchell JC. 1976. Biological activities of sesquiterpene lactones. Phytochemistry 15 , 1573–1580. ( 10.1016/S0031-9422(00)97430-2) - DOI
-
- Fu L, Palazzesi L, Pellicer J, Balant M, Christenhusz MJM, Pegoraro L, Pérez-Lorenzo I, Leitch IJ, Hidalgo O. 2023. Let’s pluck the daisy: dissection as a tool to explore the diversity of Asteraceae capitula. Bot. J. Linnean Soc. 201 , 391–399. ( 10.1093/botlinnean/boac055) - DOI
-
- Seaman FC. 1982. Sesquiterpene lactones as taxonomic characters in the Asteraceae. Bot. Rev. 48 , 121–594. ( 10.1007/BF02919190) - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

