Conserved carotenoid pigmentation in reproductive organs of Charophyceae
- PMID: 39343025
- PMCID: PMC11449214
- DOI: 10.1098/rstb.2023.0372
Conserved carotenoid pigmentation in reproductive organs of Charophyceae
Abstract
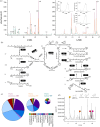
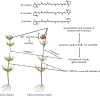
Sexual reproduction in Charophyceae abounds in complex traits. Their gametangia develop as intricate structures, with oogonia spirally surrounded by envelope cells and richly pigmented antheridia. The red-probably protectant-pigmentation of antheridia is conserved across Charophyceae. Chara tomentosa is, however, unique in exhibiting this pigmentation and also in vegetative tissue. Here, we investigated the two sympatric species, C. tomentosa and Chara baltica, and compared their molecular chassis for pigmentation. Using reversed phase C30 high performance liquid chromatography (RP-C30-HPLC), we uncover that the major pigments are β-carotene, δ-carotene and γ-carotene; using headspace solid-phase microextraction coupled to gas chromatography equipped with a mass spectrometer (HS-SPME-GC-MS), we pinpoint that the unusually large carotenoid pool in C. tomentosa gives rise to diverse volatile apocarotenoids, including abundant 6-methyl-5-hepten-2-one. Based on transcriptome analyses, we uncover signatures of the unique biology of Charophycaee and genes for pigment production, including monocyclized carotenoids. The rich carotenoid pool probably serves as a substrate for diverse carotenoid-derived metabolites, signified not only by (i) the volatile apocarotenoids we detected but (ii) the high expression of a gene coding for a cytochrome P450 enzyme related to land plant proteins involved in the biosynthesis of carotenoid-derived hormones. Overall, our data shed light on a key protection strategy of sexual reproduction in the widespread group of macroalgae. The genetic underpinnings of this are shared across hundreds of millions of years of plant and algal evolution. This article is part of the theme issue 'The evolution of plant metabolism'.
Keywords: Charophyceae; apocarotenoids; carotenoids; sexual reproductive structures; streptophyte algae.
Conflict of interest statement
We declare we have no competing interests.
Figures


Similar articles
-
A novel carotenoid cleavage activity involved in the biosynthesis of Citrus fruit-specific apocarotenoid pigments.J Exp Bot. 2013 Nov;64(14):4461-78. doi: 10.1093/jxb/ert260. Epub 2013 Sep 4. J Exp Bot. 2013. PMID: 24006419 Free PMC article.
-
A novel method for screening a vertebrate transcriptome for genes involved in carotenoid binding and metabolism.Mol Ecol Resour. 2012 Jan;12(1):149-59. doi: 10.1111/j.1755-0998.2011.03069.x. Epub 2011 Sep 27. Mol Ecol Resour. 2012. PMID: 21951614
-
Determination of Plant Volatile Apocarotenoids.Methods Mol Biol. 2020;2083:165-175. doi: 10.1007/978-1-4939-9952-1_12. Methods Mol Biol. 2020. PMID: 31745920
-
Apocarotenoids: A New Carotenoid-Derived Pathway.Subcell Biochem. 2016;79:239-72. doi: 10.1007/978-3-319-39126-7_9. Subcell Biochem. 2016. PMID: 27485225 Review.
-
Biological roles of fungal carotenoids.Curr Genet. 2015 Aug;61(3):309-24. doi: 10.1007/s00294-014-0454-x. Epub 2014 Oct 5. Curr Genet. 2015. PMID: 25284291 Review.
Cited by
-
Current and future perspectives for enhancing our understanding of the evolution of plant metabolism.Philos Trans R Soc Lond B Biol Sci. 2024 Nov 18;379(1914):20240253. doi: 10.1098/rstb.2024.0253. Epub 2024 Sep 30. Philos Trans R Soc Lond B Biol Sci. 2024. PMID: 39343013 Free PMC article.
-
Algal origins of core land plant stress response subnetworks.Plant J. 2025 Jun;122(6):e70291. doi: 10.1111/tpj.70291. Plant J. 2025. PMID: 40550109 Free PMC article.
-
Comprehensive Review on Plant Cytochrome P450 Evolution: Copy Number, Diversity, and Motif Analysis From Chlorophyta to Dicotyledoneae.Genome Biol Evol. 2024 Nov 1;16(11):evae240. doi: 10.1093/gbe/evae240. Genome Biol Evol. 2024. PMID: 39506518 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

