Growth differentiation factor 9 activates the TGF-β pathway in follicle atresia of Muscovy ducks
- PMID: 39343644
- PMCID: PMC11705381
- DOI: 10.1016/j.psj.2024.104278
Growth differentiation factor 9 activates the TGF-β pathway in follicle atresia of Muscovy ducks
Abstract
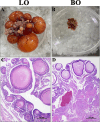
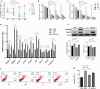
Muscovy ducks' high broodiness hinders industry growth. Studying broodiness regulation contributes to the theoretical foundation for enhancing reproductive performance in Muscovy ducks. Experiment 1, a total of 18 Muscovy ducks were divided into 2 groups: Laying group (LO) and Broody group (BO). To collect ovaries for morphological and transcriptome analysis. Experiment 2, Primary Muscovy ducks granulosa cells (GC) were isolated and treated with or without GDF9 at appropriate concentrations as indicated. Experiment 3, GC were treated with or without GDF9 in the presence or absence of a receptor inhibitor. The cell viability, cell apoptosis rate and levels of TGF-β pathway were determined. In vivo, there was a gradual disappearance of follicles in the ovaries and accompanied by follicle atrophy and a concentration of cytoplasm in BO group. The transcriptome expression profile revealed a total of 1,185 up-regulated differentially expressed transcripts (DEs) and 1,258 down-regulated DEs in the BO group compared to the LO group. The up-regulated differentially expressed GDF9 is involved in regulating the TGF-β pathway, which is among the top 10 pathways identified through the KEGG pathway analysis (P < 0.05). Additionally, the fluorescence intensity of apoptosis is primarily observed in the granulosa layers of the ovary. Compared to the LO group, the mRNA level of TGF-β pathway and the protein of GDF9 and p-Smad2/3 were increased in ovary of the BO group (P < 0.05). In vitro, GDF9 supplementation demonstrated does-related promotion of GC (P < 0.01). Compared to CTRL group, 12 ng/mL GDF9 supplementation to GC increased the rate of cell apoptosis, the mRNA and protein expression of TGF-β pathway and the apoptosis-related genes. Pretreatment of GC with GDF9-receptor inhibitor largely abrogated the negative function of GDF9 treatment (P < 0.05). In summary, granulosa cell apoptosis leading to follicle atresia in broodiness of Muscovy ducks is associated with GDF9 activation of the TGF-β pathway. This discovery lays a solid foundation for understanding duck follicular development and enhancing egg production in Muscovy ducks.
Keywords: Muscovy duck; SMDA; apoptosis; broodiness; granulosa cell.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


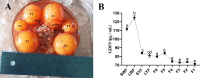



Similar articles
-
miR-317 regulates the proliferation and apoptosis of duck follicle granulosa cells by targeting VIPR1.Poult Sci. 2025 Jan;104(1):104588. doi: 10.1016/j.psj.2024.104588. Epub 2024 Nov 23. Poult Sci. 2025. PMID: 39615327 Free PMC article.
-
Investigations of TGF-β signaling in preantral follicles of female mice reveal differential roles for bone morphogenetic protein 15.Endocrinology. 2013 Sep;154(9):3423-36. doi: 10.1210/en.2012-2251. Epub 2013 Jun 19. Endocrinology. 2013. PMID: 23782946
-
Comparative Transcriptome Profiling of Ovary Tissue between Black Muscovy Duck and White Muscovy Duck with High- and Low-Egg Production.Genes (Basel). 2020 Dec 31;12(1):57. doi: 10.3390/genes12010057. Genes (Basel). 2020. PMID: 33396489 Free PMC article.
-
The role of FSH and TGF-β superfamily in follicle atresia.Aging (Albany NY). 2018 Mar 2;10(3):305-321. doi: 10.18632/aging.101391. Aging (Albany NY). 2018. PMID: 29500332 Free PMC article. Review.
-
Regulation of cell death and cell survival gene expression during ovarian follicular development and atresia.Front Biosci. 2003 Jan 1;8:d222-37. doi: 10.2741/949. Front Biosci. 2003. PMID: 12456353 Review.
References
-
- Ashburner M., Ball C.A., Blake J.A., Botstein D., Butler H., Cherry J.M., Davis A.P., Dolinski K., Dwight S.S., Eppig J.T., Harris M.A., Hill D.P., Issel-Tarver L., Kasarskis A., Lewis S., Matese J.C., Richardson J.E., Ringwald M., Rubin G.M., Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. - PMC - PubMed
-
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Royal Stat. Soc.: Series B (Methodological) 1995;57:289–300.
-
- Chang H., Brown C.W., Matzuk M.M. Genetic analysis of the mammalian transforming growth factor-beta superfamily. Endocr. Rev. 2002;23:787–823. - PubMed
-
- Drummond A.E. TGFbeta signalling in the development of ovarian function. Cell Tissue Res. 2005;322:107–115. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

